Symptomatic large bilateral adrenal metastases at presentation in small-cell lung cancer: a case report and review of the literature
Introduction
The adrenal gland is a common site for metastasis from a variety of tumors including carcinomas (lung, breast and kidney), melanoma and lymphoma (1). Adrenal metastases at the time of initial diagnosis occur in less than 10% patients with non small cell lung cancer (NSCLC) (2). The exact prevalence of adrenal metastasis in small cell lung cancer (SCLC) patients is not known. Bilateral adrenal involvement in the setting of lung cancer is not uncommon and it is usually associated with diffuse systemic spread of the primary disease (3). Large symptomatic adrenal metastases at the time of diagnosis are however exceedingly uncommon. We present a case of SCLC in whom symptomatic large adrenal metastases were diagnosed at the time of presentation.
Case report
A 46-year old male patient presented with history of discomfort and dragging sensation in the left upper abdomen of one month duration. He started experiencing non-productive cough for 15 days prior to presentation. The patient was a ‘bidi’ (this hand rolled form of tobacco wrapped in the dried tendu leaf is the commonest form of smoked tobacco in India) smoker with a 20 pack year history. The patient had sought medical attention for his abdominal discomfort. Presence of a mass lesion in relation to the upper pole of the left kidney on ultrasonography of the abdomen led to the patient being referred to our centre for further evaluation and management.
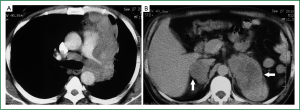
On examination at our centre, the patient was afebrile and vital signs were normal. General physical examination was unremarkable. Chest examination revealed reduced intensity of breath sounds in the left mammary area. On abdominal examination, a ballottable lump was palpable in the left hypochondrium and left lumbar regions that was mildly tender on palpation. A computed tomography (CT) scan of the thorax and abdomen was performed which demonstrated a large soft tissue mass in the upper lobe of the left lung (Figure 1A). The mass had loss of fat planes with and was encasing the pulmonary trunk and the left main pulmonary artery, extending upto the descending thoracic aorta and was accompanied by enlarged lymph nodes in the precarinal region, aorto-pulmonary window and the left hilum. The abdominal images revealed large bilateral adrenal masses (Figure 1B). The left adrenal mass measured 85 mm × 57 mm and showed ill defined fat planes and invasion of upper pole of the left kidney along with encasement of the left renal vascular pedicle. The right adrenal mass measured 34 mm × 25 mm and had intact adjoining fat planes. The adrenal masses on both sides had necrotic centres without any evidence of fat/calcification or air density.
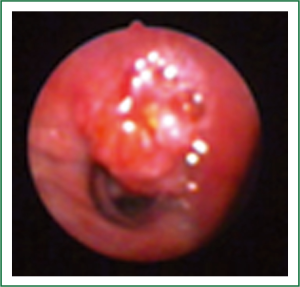
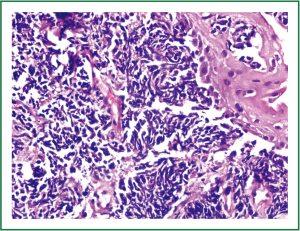
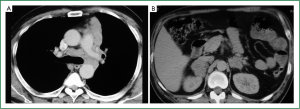
Flexible bronchoscopy (Figure 2; Supplemental video file) demonstrated mucosal infiltration in the left upper lobe bronchus extending into the left main bronchus. Bronchial biopsy confirmed the diagnosis of SCLC (Figure 3) and was supported by neuroendocrine marker positivity on immunohistochemistry. Whole body bone scan revealed increased tracer uptake in the 5th thoracic vertebra and the sacroiliac joint suggestive of bony metastases. Final diagnosis was extensive disease SCLC (T4N2M1b). The patient was started on platinum based doublet chemotherapy (irinotecan 65 mg/m2 and cisplatin 30 mg/m2 each given on days 1 and 8 of a three weekly cycle). He also received zoledronate (4 mg intravenously every four weeks) because of the presence of bony metastases. After four cycles of chemotherapy, partial response was observed on repeat evaluation (Figure 4) and an additional two cycles (total six cycles) were administered. The patient tolerated chemotherapy well and experienced only grade 1 anemia and grade 1 diarrhea which were managed with oral hematinics and supportive medications respectively. The patient experienced disease progression two months following completion of chemotherapy suggestive of refractory disease. He was lost to follow up one month later.
Discussion
Metastatic lung cancer carries a dismal prognosis and a high percentage of patients eventually die of progressive disease. Metastatic adrenal gland involvement has been reported at autopsy in upto 42% of patients with advanced NSCLC and 20% to 45% of all cancer patients (4,5). Adrenal insufficiency has also been reported as a rare feature of adrenal metastases from lung cancer (4). Uncommonly, adrenal metastases may first appear as a single synchronous or metachronous metastasis (6). Bilateral adrenal metastases occur in approximately 10% of all lung cancer patients but symptomatic large bilateral adrenal metastases are exceedingly uncommon (3). On performing a Medline search for articles in English literature, we came across only two case reports of bilateral large adrenal metastases, both of which have been reported in patients with NSCLC and in only one case, were there symptoms because of presence of the same (3,7).
There were certain salient features of our patient. First, he presented with symptomatic bilateral large adrenal masses and small cell histology - an uncommonly described clinical scenario. Second, despite the large sized bilateral adrenal lesions, the patient did not have any clinical features to suggest intratumoral hemorrhage or adrenal insufficiency either at presentation or subsequently during treatment and follow up. Third, the patient was successfully managed with conventional platinum based chemotherapy. It is worthwhile to mention here that unlike North America and elsewhere where etoposide based regimens are commonly used, this patient was treated with irinotecan-cisplatin, the standard of care for extensive disease SCLC at our centre (8). Also, the patient had a partial response on post-chemotherapy assessment by both CT as well as bronchoscopic examination.
The major concern with large adrenal metastases is adrenal hemorrhage which can be life threatening. Intratumoral hemorrhage should be promptly managed using chemotherapy or adrenalectomy (3). CT has been reported to be a sensitive and specific imaging technique for diagnosing adrenal hemorrhage and findings can vary from a heterogeneous mixed-density mass with extensive hyperdense perirenal changes to massive retroperitoneal haemorrhage (9). Successful management of massive retroperitoneal hemorrhage, a rare occurrence, with embolization has been reported (9). Retroperitoneal hemorrhage can also occur during chemotherapy. Although we cannot conclusively exclude that the necrotic appearance in the centre of the left adrenal mass in our patient was not due to intratumoral hemorrhage, the patient did not experience any sudden occurrence of symptoms or signs suggestive of such an event neither did he have a sudden fall in haemoglobin levels during the course of his presentation or subsequent follow up.
It can also be difficult to differentiate metastatic adrenal involvement from an adrenal adenoma but diagnostic accuracy for metastases increases with the presence of bilateral involvement and the simultaneous detection of the primary tumor as in our patient. Magnetic resonance imaging can be helpful in differentiating adenomas from metastases as adenomas present with low-intensity signals on T2 sequences (3). Our patient also did not have any clinical features to suggest hypo- or hyper-functioning of the adrenal cortex or medulla and hence no definitive investigations for its evaluation were performed.
An important point to be considered is that in cases, where the adrenal gland represents the solitary site of metastasis and the primary lung lesion is also resectable, adrenalectomy can be carried out along with resection of the lung tumor since such an approach can be associated with improvement in overall survival (10). Open surgical techniques are preferred in patients with metastases larger than 5 cm owing to the possible risk of local recurrence and intraperitoneal dissemination of disease with laparoscopic approaches (3).
In summary, the present case highlights the fact that patients with SCLC can rarely present with symptoms due to large bilateral adrenal gland involvement in the absence of intratumoral hemorrhage or adrenal insufficiency. Such patients can generally be managed well with conventional treatment modality for extensive disease SCLC.
Acknowledgements
Disclosure: The authors declare no conflict of Interest.
References
- Brunt LM, Moley JF. Adrenal incidentaloma. World J Surg 2001;25:905-13. [PubMed]
- Allard P, Yankaskas BC, Fletcher RH, et al. Sensitivity and specificity of computed tomography for the detection of adrenal metastatic lesions among 91 autopsied lung cancer patients. Cancer 1990;66:457-62. [PubMed]
- Karanikiotis C, Tentes AA, Markakidis S, et al. Large bilateral adrenal metastases in non-small cell lung cancer. World J Surg Oncol 2004;2:37. [PubMed]
- Mohammad K, Sadikot RT. Adrenal insufficiency as a presenting manifestation of nonsmall cell lung cancer. South Med J 2009;102:665-7. [PubMed]
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74-85. [PubMed]
- Muñoz A, López-Vivanco G, Mañé JM, et al. Metastatic non-small-cell lung carcinoma successfully treated with pre-operative chemotherapy and bilateral adrenalectomy. Jpn J Clin Oncol 2006;36:731-4. [PubMed]
- Charalambous S, Mylonaki E, Fotas A, et al. Large adrenal metastasis in non-small cell lung carcinoma. Case report and literature review. Tumori 2008;94:134-6. [PubMed]
- Singh N, Behera D. Approach to management of lung tumors. In: Jindal SK. eds. Textbook of Pulmonary and Critical Care Medicine. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd., 2011:1457-69.
- Ambika S, Melton A, Lee D, et al. Massive retroperitoneal adrenal hemorrhage secondary to lung cancer metastasis treated by adrenal artery embolization. Clin Lung Cancer 2009;10:E1-4. [PubMed]
- Beitler AL, Urschel JD, Velagapudi SR, et al. Surgical management of adrenal metastases from lung cancer. J Surg Oncol 1998;69:54-7. [PubMed]


