Minimally invasive valve surgery in high-risk patients
Introduction
Since its inception in 1996, the performance of minimally invasive valve surgery (MIVS) has grown significantly (1-3). Many different approaches for MIVS have been described, however, the most commonly utilized include a right anterior or lateral thoracotomy, an upper hemisternotomy, and the use of robotic technology (Figure 1) (4). When compared with a standard median sternotomy (ST), the reported benefits of MIVS include: reduced surgical trauma, post-operative pain, blood loss and need for re-operation for bleeding, shorter ventilation time, intensive care unit (ICU) and hospital length of stay (LOS), decreased incidence of post-operative atrial fibrillation, reduced cost, less use of rehabilitation resources, improved cosmesis, and a more rapid return to functional activity (2,5-7). Because of these advantages, high-risk patients may especially benefit from this approach, as opposed to ST (8,9). However, these potential benefits may come with an increased risk of stroke, aortic dissection or aortic injury, phrenic nerve palsy, groin infections/complications, and increased cross-clamp, cardiopulmonary bypass, and procedure time (5).
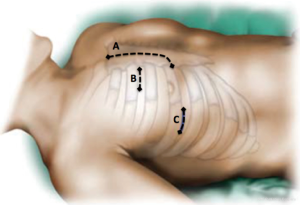
Currently, there is no clear definition of a “high-risk” patient undergoing cardiac surgery. The currently available tools of risk prediction in cardiac surgery are the EuroSCORE II (10), and The Society of Thoracic Surgeons Predicted Risk Model (11). In the present paper, when discussing “high-risk” patients, we are referring to patients perceived to be “higher-risk”, meaning those with significant comorbidity, or those undergoing procedures that are associated with an increased morbidity and mortality risk. Herein, we discuss the data of patients undergoing MIVS deemed to be a higher risk for cardiac surgery, focusing on the more recent literature.
Elderly patients
More than 4% of the US population age 75 years and older have aortic stenosis, and half of those affected will progress to hemodynamically severe aortic stenosis (12). This is a potential population of 4.2–5.6 million at present, expected to double by 2030. In regards to mitral regurgitation, prevalence in the general population increases by 1.3-fold with each decade of life, and epidemiologic studies suggest that it may be the most common valve lesion in patients age 65 years and older in the US (13,14). Despite this disease burden, approximately 33% to 49% of patients are denied surgical intervention due to a perceived age-related risk and morbidity associated with cardiac surgery (15,16).
Krishna et al., reported the outcomes of 255 consecutive patients that were ≥80 years of age and underwent MIVS for aortic valve replacement (AVR) via a right thoracotomy (17). Thirty-one (12.2%) patients had a history of prior cardiac surgery, and 3 (1.2%) required conversion to ST. Post-operatively, 4 (1.6%) patients had cerebrovascular accidents (CVAs), 8 (3.1%) had acute kidney injury (AKI), and 4 (1.6%) required re-operation for bleeding. The median hospital LOS was 7 days (IQR 6–10), with a 30-day mortality of 3.1%, demonstrating a low morbidity and mortality in those undergoing primary, or re-operative surgery. In a separate study, the outcomes of 119 patients with an age ≥75 years who underwent isolated mitral or aortic valve MIVS via a right thoracotomy were compared with 84 who had ST (18). Baseline characteristics were similar, except for a higher incidence of heart failure in patients undergoing ST (MIVS 36.1%, ST 56%, P=0.005). Three (2.5%) patients in the MIVS group required conversion to a median ST. Despite longer operative times, the MIVS group had a lower incidence of AKI (0.8% vs. 16.7%, P<0.001), prolonged mechanical ventilation (19.3% vs. 38.1%; P=0.003), and deep wound infection (0.8% vs. 6%, P=0.034), as compared with ST. The in-hospital mortality was also lower in the MIVS group, being 1.7% vs. 9.5% in the ST group, P=0.01. Though limited by its retrospective nature, this study demonstrated that when compared with ST, MIVS may reduce morbidity and mortality in elderly patients.
Iribarne et al., reported the outcomes of 175 patients who were ≥75 years of age, and underwent isolated mitral valve (MV) surgery (19). There were 70 patients who underwent MIVS via a right thoracotomy and 105 patients who underwent ST, with similar between-group baseline characteristics. The MIVS group experienced longer operative times, but with 3.1-day shorter hospitalization LOS (P=0.033), a similar rate of mitral valve repair (MVrep) (MIVS 53.5%, ST 58.9%, P=0.7), and no required conversion to ST. There were no significant differences in terms of operative mortality (7.1% vs. 2.8%, P=0.19), post-operative AKI (1.4% vs. 5.7%, P=0.16), CVA (2.9% vs. 6.7%, P=0.26), or 1-year survival (90% vs. 89.4%, P=0.9), between the MIVS vs. ST group. However, MIVS led to a reduction in re-operation for bleeding (MIVS 0, ST 4.8%, P=0.06), lower cost of hospitalization (P=0.007), more likely discharge to home (P=0.021) and faster rates of independent ambulation (P=0.039) and sit-to-stand activity (P=0.003). A propensity-matched analysis of 286 patients undergoing MV surgery, with an age >70 years, was conducted by Holzhey and colleagues (20). There were 143 patients who underwent MIVS via a right thoracotomy, and 143 who had ST. The MIVS group had longer duration of surgery (P=0.01), and a similar rate of MVrep (MIVS 53.1%, ST 58.7%, P=0.4); however, the incidence of arrhythmias (50.3% vs. 65.7%, P=0.023) and pacemaker implants (10.5% vs. 18.9%, P=0.059) was lower in the MIVS group, as compared with ST. There were no differences in the 30-day mortality (MIVS 7.7%, ST 6.3%, P=0.82) or combined major adverse cardiac and cerebrovascular events (MIVS 11.2%, ST 12.6%, P=0.86).
Left ventricular systolic dysfunction
Patients undergoing aortic or MV surgery with left ventricular systolic dysfunction have an inherently increased cardiac surgical risk, and are up to five times less likely to undergo surgical intervention, independent of other co-morbid conditions (15,16). Thus, this population may benefit the most from the reduction in surgical trauma noted with MIVS. Nevertheless, concerns over prolonged operative times have questioned the use of MIVS techniques in these patients.
Tabata and colleagues conducted a retrospective review of 140 patients with a left ventricular ejection fraction ≤40% undergoing AVR, and compared the results of 41 matched pairs who had MIVS via an upper hemisternotomy or full ST (21). The incidence of operative mortality (2.4% vs. 4.4.9%, P=0.56), AKI (0 vs. 2.4%, P=0.32), CVA (0 vs. 2.4%, P=0.32), and hospital LOS (8.5 vs. 10.6 days, P=0.17), was similar between MIVS vs. ST. Garbade et al., reported the outcomes of 177 patients with a left ventricular ejection fraction ≤30% undergoing MIVS via a right thoracotomy for mitral regurgitation (22). All patients had severe secondary mitral regurgitation, 32 (18.3%) had a history of prior cardiac surgery, and the MVrep rate was 86.4%. Post-operative complications included AKI, CVA, and re-operation for bleeding in 6.7%, 2.9%, and 6.9% of patients, respectively, and a mean hospital LOS of 17±12 days. The thirty-day mortality was 7.9%, with a ten-year survival of 45.5%. A similar study was performed in 71 patients with severe left ventricular systolic dysfunction and secondary mitral regurgitation undergoing MIVS via a right thoracotomy (23). There were 31 (44%) patients that had MVrep, and 40 (56%) that had MV replacement. No patient required conversion to ST. There was 1 (1.4%) CVA, 5 (7%) patients developed AKI, and there were no re-operations for bleeding. The median hospital LOS was 6 days (IQR 5–9 days), with an in-hospital mortality of 2.8%, which was lower than the STS predicted mortality of 6.6%. These studies demonstrate that in patients with left ventricular systolic dysfunction, MIVS can be performed safely with satisfactory perioperative outcomes.
In order to improve the durability of a MVrep for secondary mitral regurgitation, adjunctive subvalvular interventions have been increasingly utilized to correct papillary muscle displacement and relieve mitral leaflet tethering (24,25). Santana et al., analyzed the outcomes of 19 consecutive patients undergoing MIVS via a right thoracotomy for severe secondary mitral regurgitation in whom the MVrep was performed concomitantly with papillary muscle approximation utilizing a 4mm polytetrafluoroethylene graft (26,27). There were two (10.5%) cases of post-operative AKI, and no operative mortality, CVAs, or re-operations for bleeding. A subsequent study by Mihos and colleagues included a total of 58 patients who underwent MIVS MVrep and papillary muscle approximation via a right thoracotomy, which included the aforementioned cohort (28). There were no required conversions to ST, and post-operative complications included AKI in 2 (6%) patients, CVA in 1 (3%), atrial fibrillation in 2 (6%), and 30-day mortality in 2 (6%). The mean hospital LOS was 9±9 days, and actuarial survival at 1 and 3 years was 93% and 87%, respectively. These studies highlight the feasibility and safety of MIVS complex MVrep in the setting of left ventricular systolic dysfunction.
Multiple valve surgery
Double valve surgery
Double valve surgery accounts for approximately 11% of valve operations performed in the US (29). Patients requiring a multiple valve operation are typically afflicted with a more advanced disease state, which doubles the risk of operative mortality when compared with single valve operations, and decreases long-term survival (30,31). The Society of Thoracic Surgeons risk model estimates the operative mortality with combined mitral and tricuspid valve surgery at 7.6%, and with combined aortic and MV surgery at 9.4% (32).
Atik et al., performed a propensity analysis comparing the outcomes of 81 matched pairs of patients undergoing combined aortic and MV surgery via right thoracotomy MIVS or ST (33). Of note, 8.7% of MIVS and 6.2% of ST patients underwent additional tricuspid valve surgery, constituting a triple valve operation. MVrep was achieved in 62.5% of the MIVS patients, and 60.7% with a ST approach. There was no difference in operative mortality (6.2% vs. 2.5%, P=0.4), AKI (4.9% vs. 1.2%, P=0.4), CVA (2.5% vs. 2.5%, P=1.0), atrial fibrillation (65% vs. 59%, P=0.5), or re-operation for bleeding (8.6% vs. 4.9%, P=0.5), between the MIVS and ST group. Long-term survival was also similar, being 82% for MIVS and 76% for ST at 10 years (P=0.7), demonstrating that MIVS for aortic and MV surgery can be performed with results comparable to ST.
In patients undergoing combined mitral and tricuspid valve surgery, Mihos et al., retrospectively evaluated the outcomes of 132 consecutive patients who had a right thoracotomy MIVS (34). The cohort consisted of 88% primary and 12% re-operative double valve surgery, and MVrep was performed in 67%. Post-operatively, there were 6 (5%) cases of AKI, 6 (5%) re-operations for bleeding, 4 (3%) CVAs, and 12 (9%) developed atrial fibrillation. The median hospital LOS was 8 days (IQR 6–13 days), and the in-hospital mortality was 4%. Survival at 1 and 5 years was 93% and 88%, respectively. Another retrospective study reported the outcomes of 441 patients who had combined mitral and tricuspid valve surgery via a right thoracotomy over a 10-year period, of which 7.3% underwent re-operative double valve surgery (35). The rate of MVrep was 79.8%. Post-operatively, there were 37 (8.2%) re-operations for bleeding, 10 (2.3%) CVAs, and 19 (4.3%) deaths at 30 days. The actuarial survival at 5 years was 77.2%, demonstrating that MIVS for mitral and tricuspid valve can be performed routinely with good long-term outcomes.
Re-operative valve surgery
Re-operations pose a significant challenge due to the presence of pleural and epicardial adhesions, patent bypass grafts from previous coronary artery bypass graft surgery, which are at risk of intraoperative injury, and more challenging myocardial protection related to coronary artery disease and arterial bypass grafts. The operative mortality of patients undergoing re-operative valve surgery via ST has been reported as 11.1% to 13.4%, with each sternal re-entry conferring a 2.6-fold increased risk of death (36,37). Thus, the avoidance of sternal re-entry, limited dissection of adhesions, and avoiding the risk of injury to cardiac structures or patent grafts by utilizing MIVS is appealing in patients undergoing re-operation.
Reoperative aortic valve surgery
Kaneko et al., compared the results of 51 octogenarians undergoing re-operative AVR via an upper hemisternotomy MIVS approach with 54 who underwent ST (38). Both groups had similar baseline characteristics. There was no difference in post-operative complications between the MIVS and ST group, including operative mortality (3.9% vs. 9.3%, P=0.44), AKI (0 vs. 7.4%, P=0.12), CVA (5.9% vs. 1.9%, P=0.35), re-operation for bleeding (7.8% vs. 3.7%, P=0.43), and atrial fibrillation (15.7% vs. 28.7%, P=0.16). Actuarial survival was higher for MIVS at both 1 year (92% vs. 79%) and 5 years (65% vs. 38%, P=0.028) post-operatively, as compared with ST. Another study of 77 patients undergoing re-operative AVR, compared the results of 36 patients who underwent right thoracotomy MIVS with 41 patients that had ST (39). The MIVS group consisted of older patients (MIVS 75.3±9, ST 68.2±13.6 years, P=0.009), and a higher prevalence of previous coronary artery bypass graft surgery (MIVS 86%, ST 59%, P=0.007). Composite post-operative complications occurred in 17% vs. 46% (P=0.005), and the ICU and hospital LOS were 48 (IQR, 41–97) vs. 69 hours (IQR, 45–174) (P=0.03) and 7 (IQR, 5–10) vs. 9 days (IQR, 7–15) (P=0.03), for the MIVS vs. ST group, respectively. The in-hospital mortality was 0 for patients undergoing MIVS cohort vs. 4 (10%) who received a ST, P=0.08.
A systematic review of 13 retrospective studies analyzed the results of MIVS and ST for patients undergoing re-operative AVR (40). The results demonstrated that for patients with prior cardiac surgery undergoing re-operative AVR, MIVS by means of a right thoracotomy or a partial upper hemisternotomy is feasible, and is at least as safe as conventional ST approach. It is associated with shorter hospital LOS and less blood product requirements, and may offer better post-operative outcomes, including decreased mechanical ventilation time, sternal wound infection, bleeding requiring re-operation, and in-hospital mortality.
Reoperative mitral surgery
Murzi et al., reported the outcomes of 173 right thoracotomy MIVS performed for mitral regurgitation in patients with a previous ST (41). MVrep was achieved in 30.6% of cases, and there were 2 (1.1%) required conversions to ST. The mean blood transfusion requirement was 1.4±1.1 units. Major complications included 11 (6%) CVAs, 11 (6.3%) re-operations for bleeding, and 4 (2.3%) developed AKI requiring dialysis. Thirty-day mortality occurred in 7 (4.1%) patients, and survival at 1, 5, and 10 years was 93.1%, 87.5%, and 79.7%, respectively. The authors concluded that avoidance of extensive surgical dissection, optimal valve exposure, and low blood transfusion requirements were the main advantages of this technique. Mihos et al., compared the outcomes of 59 patients with mitral regurgitation undergoing re-operative right thoracotomy MIVS with 29 that underwent repeat ST (42). The patients in the MIVS group had a higher incidence of diabetes mellitus (MIVS 34%, ST 10%, P=0.02) and prior myocardial infarction (MIVS 37%, ST 17%, P=0.06), while more patients undergoing ST had a history of congestive heart failure (MIVS 46%, ST 76%, P=0.008). MVrep was more commonly performed in the MIVS group (MIVS 42%, ST 17%, P=0.02), and no patient undergoing MIVS required conversion to ST. The composite post-operative complications occurred in 29% of the MIVS group and 66% of the ST group (P=0.001), which was driven by a reduction in the incidence of prolonged mechanical ventilation (MIVS 15%, ST 55%, P<0.001). The median ICU and hospital LOS were [48 (IQR, 41–90) vs. 118 (IQR, 67–167) hours, P<0.001] and [8 (IQR, 6–12) vs. 13 (IQR, 9–18) days, P=0.001] for the MIVS vs. ST group, respectively. Finally, operative mortality occurred in 2 (3%) in the MIVS and 4 (14%) in the ST group, P=0.07.
A systematic review analyzed nine retrospective studies comparing MIVS for re-operative MV surgery with a repeat ST approach. The authors concluded that the peri-operative morbidity and mortality was similar between the two surgical approaches (43). The main advantages of re-operative MIVS were less post-operative bleeding, reduced need for blood transfusions, absence of deep sternal wound infections, and a higher rate of patient satisfaction. Furthermore, it appeared that hospital LOS and ventilation times were also reduced in these patients.
Multiple reoperations
Patients requiring multiple re-operations are a significantly high-risk group, not only because of comorbidity, but also the increased amount of adhesions and bypass grafts. As noted earlier, each sternal re-entry after the index ST more than doubles the risk of operative mortality, and thus, it may be that this group would benefit the most from MIVS (36). A retrospective review of the outcomes of MIVS via a right thoracotomy in 38 consecutive patients with a prior history of two or more cardiac surgeries, including coronary artery bypass graft and/or valve surgery was performed (44). Eighteen (47%) patients had at least two prior valve surgeries, 11 (29%) had both previous coronary artery bypass graft and valve surgery, and 9 (24%) had two prior coronary artery bypass graft operations, There were 34 isolated valve procedures, consisting of 24 MV operations, 9 AVRs, and 1 tricuspid valve repair; there were 4 patients that underwent combined mitral and tricuspid valve surgery. Post-operative complications included 2 (5.3%) CVAs, 3 (7.9%) re-operations for bleeding, and 3 (7.9%) developed AKI. The median hospital LOS was 9.5 days (IQR, 7–16 days), and 30-day mortality occurred in 3 (7.9%) patients. The cumulative survival at 1 and 5 years was 82% and 72%, respectively. This study demonstrated that MIVS after multiple prior cardiac operations, for isolated or double valve procedures, is associated with acceptable peri-operative outcomes and mid-term survival.
Obese patients
A high body mass index in patients undergoing cardiac surgery is associated with an increased risk of peri-operative morbidity, including deep sternal wound infections, respiratory and renal complications, and prolonged ICU and hospital LOS (45-47). Consequently, obesity has often been considered a contraindication to MIVS, owing to the surgical risk and concerns regarding adequate surgical field exposure, lack of outcome data, and operator inexperience (48).
Santana et al., reported the results of 160 consecutive obese patients with a body mass index >30 kg/m2 who underwent isolated mitral and/or aortic valve surgery (49). The outcomes of 64 patients that underwent MIVS via a right thoracotomy were compared with 96 who had ST. The baseline characteristics were similar between groups, with the exception that the MIVS group was older (MIVS 69.4±11, ST 64.7±11.5 years, P=0.015). The MIVS group experienced longer operative times; however, no patient required conversion to ST for inadequate surgical field exposure. When compared with ST, the composite of post-operative complications, which were defined as the presence of postoperative renal failure, prolonged ventilation, reintubation, deep wound infection, pneumonia, sepsis, bleeding requiring re-exploration, stroke, or death, occurred significantly less in patients undergoing MIVS (23.5% vs. 51%, P=0.034), with a lower incidence of AKI (0 vs. 6.3%, P=0.041), prolonged mechanical ventilation (18.7% vs. 34.3%, P=0.049), and need for reintubation (4.7% vs. 15.6%, P=0.032). Additionally, a MIVS approach was associated with a reduction in the hospital LOS [7.7 (IQR, 5–9) vs. 11.7 (IQR, 7–16) days, P<0.001] and 30-day mortality (0 vs. 8.3%, P=0.041), as compared with ST. This study demonstrated not only that MIVS is feasible in the obese, but that it is associated with a significant reduction in morbidity and mortality when compared with ST, despite longer operative times.
In a study that evaluated the effects of obesity on the outcomes of minimally invasive MV surgery via a right thoracotomy, Reser et al., compared the outcomes of 27 obese patients with body mass index ≥30 kg/m2 with 108 patients who had a normal weight with a body mass index lower than 25 kg/m2, and 90 patients who were overweight with a body mass index of 25 to 29 kg/m2 (50). The rate of required conversion to ST was similar between groups (obese 11.1%, non-obese 2%, P=0.08), as was the performance of MVrep (obese 85.2%, non-obese 93.9%, P=0.18). There were no differences in the occurrence of major adverse cardiac and cerebrovascular events; however, the obese patients did have longer ventilation times. Operative mortality occurred in 1 (0.5%) patient who was non-obese, and no patients in the obese group, P=1.0. Hospital lengths of stay, early survival, and freedom from re-operation were comparable between groups. The authors concluded that obesity should not deter a surgeon from selecting MIVS.
Chronic obstructive pulmonary disease (COPD)/pulmonary hypertension
The prevalence of COPD is estimated to be as high as 27% in patients undergoing cardiac surgery (51,52). After ST, there is the development of diaphragmatic dysfunction, with a reduction in vital capacity of 55% and functional residual capacity of 30% (53-55). These effects are exacerbated in patients with underlying COPD and contribute to development of atelectasis, pneumonia, and ventilation/perfusion mismatching. It is hypothesized that the improved thoracic stability noted with MIVS, as well as less surgical trauma and decreased mechanical ventilation time, leads to earlier mobilization and normalization of pulmonary function.
In a study of 165 patients with COPD who had isolated aortic or MV surgery, Santana et al., compared the results of 100 patients who underwent MIVS via a right thoracotomy with 65 that had ST (56). The baseline characteristics did not differ between the two groups. In patients undergoing MIVS, the COPD severity was mild, moderate, or severe in 15%, 82%, and 3% of the patients, while in the ST group, 19% were mild, 75% were moderate, and 6% were severe (P=0.49). Amongst patients undergoing MV surgery, a valve repair was achieved in 33% of MIVS patients vs. 40% of those undergoing ST (P=0.57). There were no required conversions to ST in patients undergoing MIVS, and the incidence of operative mortality was similar (MIVS 1%, ST 5%, P=0.14). The composite post-operative complications were reduced in the MIVS group (30% vs. 54%, P=0.002), which was driven by less prolonged mechanical ventilation (13% vs. 28%, P=0.02), as compared with ST. Finally a MIVS approach decreased the ICU and hospital LOS by 24 hours and 3 days respectively (P<0.001, for both).
As in patients with COPD, the presence of pre-operative pulmonary hypertension increases the surgical risk of cardiac surgery. Peri-operative complications occur in approximately 14% of patients with pulmonary hypertension, and this risk increases commensurate to the severity of the disease (57). The outcomes of a right thoracotomy MIVS for aortic and/or MV surgery was analyzed in 569 patients with pulmonary hypertension, with a mean pre-operative pulmonary artery pressure of 33±8 mmHg (58). The pulmonary hypertension severity was mild in 48% of the patients, moderate in 35%, and severe in 17%. There were 76 (13%) combined mitral and aortic valve operations, and no patient required conversion to ST. Operative mortality occurred in 20 (3.5%) patients, 8 (1.4%) had a CVA, and the mean ICU and hospital LOS were 50±14 hours and 7±1 days, respectively. Despite a longer mechanical ventilation time and ICU LOS, patients with severe pulmonary hypertension (mean pulmonary artery pressure ≥40 mmHg) had similar outcomes to those with mild or moderate disease, highlighting the safety and feasibility of MIVS in this population.
Chronic kidney disease
Patients with chronic kidney disease account for approximately 10% of the US population, and may have a greater than 5-fold increased risk of mortality when undergoing cardiac surgery (59-61). These poor outcomes are the result of chronic uremic and metabolic derangements that occur with long-standing renal impairment, as well as upregulation of the inflammatory cascade, systemic endothelial dysfunction, and abnormal platelet function (59). Further deterioration of renal function after cardiac surgery is associated with prolonged hospital LOS, with even minor increases in serum creatinine negatively impacting resource utilization (62).
Valdez et al., evaluated the outcomes of 688 patients with chronic kidney disease stages 2–5, who had isolated aortic or MV surgery (63). The results of 510 who had MIVS via a right thoracotomy were compared with 178 who had ST. Baseline characteristics were similar, with the exception of older age in the MIVS group (MIVS 71.3±11.6 years, ST 67.8±12 years, P=0.001). In patients undergoing MV surgery, a valve repair was performed in 60% in the MIVS group, and 66% of ST patients (P=0.36). Operative mortality occurred in 7 (1%) patients in the MIVS group, and 6 (3%) who underwent a ST (P=0.17). MIVS was associated with a lower incidence of AKI (8% vs. 15%, P=0.01), prolonged mechanical ventilation (17% vs. 28%, P=0.002) and reintubation (6% vs. 13%, P=0.004), sepsis (2% vs. 6%, P=0.008), and atrial fibrillation (19% vs. 27%, P=0.02), as compared with ST. With a MIVS approach, the ICU and hospital LOS was reduced by 23 hours and 2 days, respectively.
In a propensity analysis by Tang et al., the outcomes of 90 matched pairs of patients who underwent MV surgery via a MIVS right thoracotomy vs. ST were evaluated (64). All patients had chronic kidney disease, with pre-operative creatinine of 1.3 mg/dL or greater. MVrep was performed in 36% of MIVS patients, and 39% in the ST group, P=0.64. The patients undergoing MIVS had a lower incidence of AKI (10% vs. 21%, P=0.05), CVA (1% vs. 9%, P=0.017), and pacemaker insertion (3% vs. 11%, P=0.044), as compared with ST. While the operative mortality was similar between the groups (MIVS 6%, ST 10%, P=0.28), a MIVS approach was associated with a 20% greater actuarial survival at mid to long-term follow-up (P=0.037).
MV infective endocarditis
The incidence of infective endocarditis is cited at 1.4 to 6.2 cases per 100,000 person years, with the MV affected in approximately two-thirds of patients with left-sided valvular endocarditis (65,66). Despite advancements in medical and surgical therapy, morbidity remains high, with 10-year overall and event-free survival reported to be 50% and 17%, respectively. When considering surgical intervention, MVrep is favored over replacement given its association with superior short- and long-term outcomes (67,68).
Mihos et al., reported their outcomes of 50 patients with native MV endocarditis undergoing surgical intervention (69). The results of 22 patients who had MIVS via a right thoracotomy were compared with 28 patients who had ST. There were no baseline differences noted between groups, and no patient undergoing MIVS required a conversion to ST. Disease burden, as measured by the presence of chordal rupture, annular abscess, cusp perforation, and vegetation size, was similar between the groups. A MIVS approach was associated with a decreased incidence of sepsis (0 vs. 21%, P=0.02), less use of intra-operative blood products (59% vs. 93%, P=0.004), higher rates of MVrep (56% vs. 21%, P=0.02), and shorter ICU LOS (56 vs. 114 hours, P=0.009), as compared with ST. There was no difference in in-hospital mortality (5% vs. 14%, P=0.25), or 2.5-year actuarial survival (80% vs. 68%, P=0.33), between a MIVS vs. ST approach. This study, though limited by its small size and retrospective nature, did demonstrate a significant reduction in post-operative complications, as well as higher rates of MVrep, with MIVS.
Conclusions
The data on MIVS demonstrate that, in higher-risk patients, adequate exposure of the surgical field can be obtained with experienced surgical teams. Additionally, this approach is associated with a lower use of blood products, shorter LOS, a lower morbidity, and in some instances a lower mortality, when compared with ST. However, the available evidence concerning the outcomes of MIVS in higher-risk patients is limited, due to the fact that the results were mainly derived from single center, retrospective studies, and thus are subject to significant selection bias. Also, the cohorts tended to be small in size, and provided only short-term outcomes. The same limitations apply to the studies that compared MIVS with ST. Finally, the advent of percutaneous therapies for patients at a prohibitive surgical risk has expanded the armamentarium available to surgeons and cardiologists in the treatment of valvular heart disease, and how the clinical outcomes of these newer techniques compare to MIVS is largely unknown (70,71). Adequately powered randomized prospective trials comparing MIVS with ST in higher-risk patients, as well as with transcatheter valvular therapies, are needed to corroborate these conclusions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Navia JL, Cosgrove DM 3rd. Minimally invasive mitral valve operations. Ann Thorac Surg 1996;62:1542-4. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Goldstone AB, Joseph Woo Y. Minimally invasive surgical treatment of valvular heart disease. Semin Thorac Cardiovasc Surg 2014;26:36-43. [Crossref] [PubMed]
- Langer NB, Argenziano M. Minimally invasive cardiovascular surgery: incisions and approaches. Methodist Debakey Cardiovasc J 2016;12:4-9. [Crossref] [PubMed]
- Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [Crossref] [PubMed]
- Santana O, Larrauri-Reyes M, Zamora C, et al. Is a minimally invasive approach for mitral valve surgery more cost-effective than median sternotomy? Interact Cardiovasc Thorac Surg 2016;22:97-100. [Crossref] [PubMed]
- Mihos CG, Santana O, Lamas GA, et al. Incidence of post-operative atrial fibrillation in patients undergoing minimally invasive versus median sternotomy valve surgery. J Thorac Cardiovasc Surg 2013;146:1436-41. [Crossref] [PubMed]
- Schmitto JD, Mohr FW, Cohn LH. Minimally invasive aortic valve replacement: how does this perform in high-risk patients? Curr Opin Cardiol 2011;26:118-22. [Crossref] [PubMed]
- Moscarelli M, Fattouch K, Casula R, et al. What is the role of minimally invasive mitral valve surgery in high-risk patients? A meta-analysis of observational studies. Ann Thorac Surg 2016;101:981-9. [Crossref] [PubMed]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734-44; discussion 744-5. [Crossref]
- O’Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2-isolated valve surgery. Ann Thorac Surg 2009;88:S23-42. [Crossref] [PubMed]
- Cosmi JE, Kort S, Tunick PA, et al. The risk of the development of aortic stenosis in patients with “benign” aortic valve thickening. Arch Intern Med 2002;162:2345-7. [Crossref] [PubMed]
- Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999;83:897-902. [Crossref] [PubMed]
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005;26:2714-20. [Crossref] [PubMed]
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65. [Crossref] [PubMed]
- Krishna RK, Santana O, Mihos CG, et al. Minimally invasive aortic valve replacement in octogenarians performed via a right anterior thoracotomy approach. J Heart Valve Dis 2014;23:671-4. [PubMed]
- Lamelas J, Sarria A, Santana O, et al. Outcomes of minimally invasive valve surgery versus median sternotomy in patients 75 years or greater. Ann Thorac Surg 2011;91:79-84. [Crossref] [PubMed]
- Iribarne A, Easterwood R, Russo MJ, et al. Comparative effectiveness of minimally invasive versus traditional sternotomy mitral valve surgery in elderly patients. J Thorac Cardiovasc Surg 2012;143:S86-90. [Crossref] [PubMed]
- Holzhey DM, Shi W, Borger MA, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg 2011;91:401-5. [Crossref] [PubMed]
- Tabata M, Aranki SF, Fox JA, et al. Minimally invasive aortic valve replacement in left ventricular dysfunction. Asian Cardiovasc Thorac Ann 2007;15:225-8. [Crossref] [PubMed]
- Garbade J, Seeburger J, Merk DR, et al. Mitral valve pathology in severely impaired left ventricles can be successfully managed using a right-sided minimally invasive surgical approach. Eur J Cardiothorac Surg 2013;44:e1-7. [Crossref] [PubMed]
- Santana O, Reyna J, Pineda AM, et al. Outcomes of minimally invasive mitral valve surgery in patients with an ejection fraction of 35% or less. Innovations (Phila) 2013;8:1-5. [Crossref] [PubMed]
- Mihos CG, Yucel E, Santana O. The role of papillary muscle approximation in mitral valve repair for the treatment of secondary mitral regurgitation. Eur J Cardiothorac Surg 2017;51:1023-30. [Crossref]
- Mihos CG, Larrauri-Reyes M, Santana O. A meta-analysis of ring annuloplasty versus combined ring annuloplasty and subvalvular repair for moderate-to-severe functional mitral regurgitation. J Card Surg 2016;31:31-7. [Crossref] [PubMed]
- Santana O, Solenkova NV, Pineda AM, et al. Minimally invasive papillary muscle sling placement during mitral valve repair in patients with functional mitral regurgitation. J Thorac Cardiovasc Surg 2014;147:496-9. [Crossref] [PubMed]
- Lamelas J, Mihos C, Santana O. Surgical technique: papillary muscle sling for functional mitral regurgitation during minimally invasive valve surgery. Heart Surg Forum 2013;16:E295-7. [Crossref] [PubMed]
- Mihos CG, Capoulade R, Yucel E, et al. Combined papillary muscle sling and ring annuloplasty for moderate-to-severe secondary mitral regurgitation. J Card Surg 2016;31:664-71. [Crossref] [PubMed]
- Lee R, Li S, Rankin JS, et al. Fifteen-year outcome trends for valve surgery in North America. Ann Thorac Surg 2011;91:677-84. [Crossref] [PubMed]
- LaPar DJ, Mulloy DP, Stone ML, et al. Concomitant tricuspid valve operations affect outcomes after mitral operations: a multiinstitutional, statewide analysis. Ann Thorac Surg 2012;94:52-7. [Crossref] [PubMed]
- Vassileva CM, Li S, Thourani VH, et al. Outcome characteristics of multiple-valve surgery: comparison with single-valve procedures. Innovations (Phila) 2014;9:27-32. [Crossref] [PubMed]
- Rankin JS, He X, O'Brien SM, et al. The Society of Thoracic Surgeons risk model for operative mortality after multiple valve surgery. Ann Thorac Surg 2013;95:1484-90. [Crossref] [PubMed]
- Atik FA, Svensson LG, Blackstone EH, et al. Less invasive versus conventional double-valve surgery: a propensity-matched comparison. J Thorac Cardiovasc Surg 2011;141:1461-8. [Crossref] [PubMed]
- Mihos CG, Pineda AM, Davila H, et al. Combined mitral and tricuspid valve surgery performed via a right minithoracotomy approach. Innovations (Phila) 2015;10:304-8. [Crossref] [PubMed]
- Pfannmüller B, Davierwala P, Hirnle G, et al. Concomitant tricuspid valve repair in patients with minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:758-64. [PubMed]
- Toker ME, Eren E, Guler M, et al. Second and third cardiac valve re-operations: Factors influencing death and long-term survival. Tex Heart Inst J 2009;36:557-62. [PubMed]
- Balsam LB, Grossi EA, Greenhouse DG, et al. Re-operative valve surgery in the elderly: Predictors of risk and long-term survival. Ann Thorac Surg 2010;90:1195-200. [Crossref] [PubMed]
- Kaneko T, Loberman D, Gosev I, et al. Re-operative aortic valve replacement in the octogenarians—minimally invasive technique in the era of transcatheter valve replacement. J Thorac Cardiovasc Surg 2014;147:155-62. [Crossref] [PubMed]
- Pineda AM, Santana O, Reyna J, et al. Outcomes of aortic valve replacement via right mini-thoracotomy versus median sternotomy. J Heart Valve Dis 2013;22:50-5. [PubMed]
- Pineda AM, Santana O, Lamas GA, Lamelas J. Is a minimally invasive approach for re-operative aortic valve replacement superior to standard full resternotomy? Interact Cardiovasc Thorac Surg 2012;15:248-52. [Crossref] [PubMed]
- Murzi M, Miceli A, Di Stefano G, et al. Minimally invasive right thoracotomy approach for mitral valve surgery in patients with previous sternotomy: a single institution experience with 173 patients. J Thorac Cardiovasc Surg 2014;148:2763-8. [Crossref] [PubMed]
- Mihos CG, Santana O, Lamas GA, et al. Outcomes of right mini-thoracotomy mitral valve surgery in patients with previous sternotomy. Ann Thorac Surg 2011;91:1824-7. [Crossref] [PubMed]
- Murzi M, Solinas M, Glauber M. Is a minimally invasive approach for re-operative mitral valve surgery superior to standard resternotomy? Interact CardioVasc Thorac Surg 2009;9:327-32. [Crossref] [PubMed]
- Santana O, Krishna RK, Kherada N, et al. Outcomes of minimally invasive re-operative valve surgery in patients with multiple previous cardiac operations. J Heart Valve Dis 2016;25:487-90. [PubMed]
- Virani SS, Nambi V, Lee VV, et al. Obesity: an independent predictor of in-hospital post-operative renal insufficiency among patients undergoing cardiac surgery? Tex Heart Inst J 2009;36:540-5. [PubMed]
- Kuduvalli M, Grayson AD, Oo AY, et al. Risk of morbidity and in-hospital mortality in obese patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg 2002;22:787-93. [Crossref] [PubMed]
- Prabhakar G, Haan CK, Peterson ED, et al. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons’ database. Ann Thorac Surg 2002;74:1125-30. [Crossref] [PubMed]
- Gammie JS, Bartlett ST, Griffith BP. Small-incision mitral valve repair. Safe, durable, and approaching perfection. Ann Surg 2009;250:409-15. [PubMed]
- Santana O, Reyna J, Grana R, et al. Outcomes of minimally invasive valve surgery versus standard sternotomy in obese patients undergoing isolated valve surgery. Ann Thorac Surg 2011;91:406-10. [Crossref] [PubMed]
- Reser D, Sündermann S, Grünenfelder J, et al. Obesity should not deter a surgeon from selecting a minimally invasive approach for mitral valve surgery. Innovations (Phila) 2013;8:225-9. [Crossref] [PubMed]
- Gardner SC, Grunwald GK, Rumsfeld JS, et al. Risk factors for intermediate-term survival after coronary artery bypass grafting. Ann Thorac Surg 2001;72:2033-7. [Crossref] [PubMed]
- Leavitt BJ, Ross CS, Spence B, et al. Long-term survival of patients with chronic obstructive pulmonary disease undergoing coronary artery bypass surgery. Circulation 2006;114:I430-4. [Crossref] [PubMed]
- Weissman C. Pulmonary function after cardiac and thoracic surgery. Anesth Analg 1999;88:1272-9. [Crossref] [PubMed]
- Meyers JR, Lembeck L, O’Kane H, et al. Changes in functional residual capacity of the lung after operation. Arch Surg 1975;110:576-83. [Crossref] [PubMed]
- Craig DB. Postoperative recovery of pulmonary function. Anesth Analg 1981;60:46-52. [Crossref] [PubMed]
- Santana O, Reyna J, Benjo AM, et al. Outcomes of minimally invasive valve surgery in patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2012;42:648-52. [Crossref] [PubMed]
- Kennedy JL, LaPar DJ, Kern JA, et al. Does the Society of Thoracic Surgeons risk score accurately predict operative mortality for patients with pulmonary hypertension? J Thorac Cardiovasc Surg 2013;146:631-7. [Crossref] [PubMed]
- Gosain P, Larrauri-Reyes M, Mihos CG, et al. Aortic and/or mitral valve surgery in patients with pulmonary hypertension performed via a minimally invasive approach. Interact Cardiovasc Thorac Surg 2016;22:668-70. [Crossref] [PubMed]
- Howell NJ, Keogh BE, Bonser RS, et al. Mild renal dysfunction predicts in-hospital mortality and post-discharge survival following cardiac surgery. Eur J Cardiothorac Surg 2008;34:390-5. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2014. Atlanta, GA: US Department of Health and Human Services (HHS), CDC; 2014.
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003;42:1050-65. [Crossref] [PubMed]
- Dasta JF, Kane-Gill SL, Durtschi AJ, et al. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008;23:1970-4. [Crossref] [PubMed]
- Valdez GD, Mihos CG, Santana O, et al. Incidence of post-operative acute kidney injury in patients with chronic kidney disease undergoing minimally invasive valve surgery. J Thorac Cardiovasc Surg 2013;146:1488-93. [Crossref] [PubMed]
- Tang P, Onaitis M, Desai B, et al. Minithoracotomy versus sternotomy for mitral surgery in patients with chronic renal impairment: a propensity-matched study. Innovations (Phila) 2013;8:325-31. [Crossref] [PubMed]
- Bayer AS. Infective endocarditis. Clin Infect Dis 1993;17:313-20. [Crossref] [PubMed]
- Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest 2007;132:1025-35. [Crossref] [PubMed]
- Evans CF, Gammie JS. Surgical management of mitral valve infective endocarditis. Semin Thorac Cardiovasc Surg 2011;23:232-40. [PubMed]
- Feringa HH, Shaw LJ, Poldermans D, et al. Mitral valve repair and replacement in endocarditis: a systematic review of literature. Ann Thorac Surg 2007;83:564-70. [Crossref] [PubMed]
- Mihos CG, Santana O, Pineda AM, et al. Minimally invasive surgery for mitral valve infective endocarditis. J Heart Valve Dis 2014;23:343-9. [PubMed]
- Vahl TP, Kodali SK, Leon MB. Transcatheter Aortic Valve Replacement 2016: A Modern-Day “Through the Looking-Glass” Adventure. J Am Coll Cardiol 2016;67:1472-87. [Crossref] [PubMed]
- Testa L, Latib A, Montone RA, et al. Transcatheter mitral valve regurgitation treatment: State of the art and a glimpse to the future. J Thorac Cardiovasc Surg 2016;152:319-27. [Crossref] [PubMed]


