High expression of PLA2G16 is associated with a better prognosis in HER2-positive breast cancer
Introduction
Breast cancer is the most frequently diagnosed cancer worldwide, accounting for 30% of total cancer cases and 14% of cancer deaths according to cancer statistics from 2017 (1). Moreover, the decrease in age of onset and the increase in incidence have made breast cancer the most serious health threat to women in China. Breast cancer is a heterogeneous disease which was classified into several subtypes based on the IHC staining of ER, PR, HER2 (2) and Ki-67 (3). Although the utilization of new drugs targeting ER or HER2 has significantly improved the 5-year survival rates (4), drug resistance remains a challenge to overcome (5). Therefore, more efforts should be taken to identify new biomarkers for drug resistant breast cancer.
PLA2G16 (Group XVI phospholipase A2) was first classified as a tumor suppressor in H-RAS-resistant murine fibroblasts (6-8). Additionally, PLA2G16 expression was downregulated in miscellaneous tumorigenic cell lines derived from melanoma, neuroblastoma, adenocarcinoma, and fibrosarcoma and was absent in several primary tumors (9-11). However, PLA2G16 was later identified as a member of the phospholipase A2 family, which catalyzes the release of free fatty acids (FFAs) and lysophospholipid (LPA) from phosphatidylcholine by hydrolyzing the ester bond at the sn-2 position (6). Both LPA and FFA are known to contribute to tumorigenesis and progression (12,13). Notably, high expression of PLA2G16 was associated with poor prognosis in non-small cell lung cancer patients, and PLA2G16 overexpression promoted proliferation and metastatic potential in a subset of osteosarcoma cell lines and patients (14,15). Thus, PLA2G16 can also be classified as an oncogene, and it is likely that the role of PLA2G16 in tumor progression is tumor type-specific.
However, the PLA2G16 expression status and its clinical significance in patients with breast cancer remains elusive. Here, we examined the expression profile of PLA2G16 in 200 stage I to III invasive ductal carcinoma specimens and evaluated its association with the clinicopathological characteristics and prognosis of breast cancer patients.
Materials and methods
Patients and specimens
In total, 200 female patients diagnosed with stage I to III unilateral invasive ductal carcinoma were enrolled in the cohort. The specimens were obtained from the Department of Breast Surgery at the Fudan University Shanghai Cancer Center (FUSCC, Shanghai, China) between March 2003 and January 2008. These patients underwent a mastectomy and axillary lymph node dissection or breast conservation surgery. The therapeutic regimen decisions were based on the Chinese Anti-Cancer Association guidelines for the diagnosis and treatment of breast cancer. In our cohort, these patients were regularly followed-up. The study was approved by the Review Board of FUSCC, and informed consent forms were signed by each participant. The number/ID of the Ethics Approval is 050432-4-1212B.
Breast cancer tissue microarray construction
Formalin-fixed and paraffin-embedded tumor specimens were punched in duplicate to obtain 2 mm diameter tissue cylinders from the tumor area, which was identified by hematoxylin and eosin staining. The samples were transferred to recipient array blocks using a tissue microarrayer (TMA). The TMAs in this study were generated by the Department of Pathology at FUSCC.
Immunohistochemical staining for PLA2G16
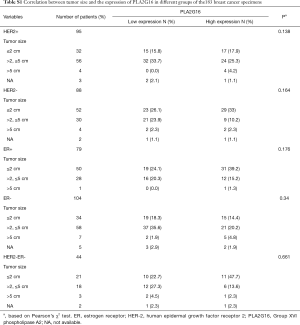
The expression of PLA2G16 was determined in the TMAs. The TMAs were dewaxed in xylene, hydrated in descending grades of ethanol and washed. Antigen retrieval was performed with a high-voltage protocol in 0.01 mol/L sodium citrate buffer for 150 s. Subsequently, tissues were incubated overnight at 4 °C with a rabbit anti-human AdPLA2 (PLA2G16) polyclonal primary antibody (No. 10337, Cayman Chemical) at a concentration of 5 µg/mL, followed by incubation with horseradish peroxidase-conjugated secondary antibodies at 37 °C for 20 minutes. Tissues were stained with diaminobenzidine solution under a microscope and counterstained with hematoxylin. The immunostaining evaluation was independently conducted by two experienced pathologists. PLA2G16 expression was scored based on the signal intensity (0= no staining, 1= weak staining, 2= moderate staining, 3= strong staining). Tissues with an immunohistochemical score of 2 or less were considered as low expression, and those with a score of 3 were considered as high expression (or overexpression).
Patients cohort for Kaplan-Meier plotter analysis
We investigated the relationship between PLA2G16 expression and clinical outcome using Kaplan-Meier Plotter, a large population-based database, with an auto-select best cutoff. In the present study, we evaluated the association between PLA2G16 expression and disease-free survival (DFS) in all breast cancer patients (3,554 cases), overall survival (OS) in all breast cancer patients (1,117 cases) and DFS in HER2-positive breast cancer patients (168 cases). All analyses were performed using the latest version of the Kaplan-Meier Plotter database (2014 version; http://www.kmplot.com/analysis/index.php?p=service).
Statistical analyses
The follow-up information was collected from surgery to the last observation. DFS was defined as the time from the date of primary surgery to the date of relapse, breast cancer-specific death or the end of the study. OS was defined as the time from primary surgery to the date of death. Patients without events or death were regarded as censored cases. The IHC staining was performed to evaluate the expression status of PLA2G16. The Kaplan-Meier analysis and log-rank test were used to evaluate the prognostic value. The Multivariate Cox regression analysis was performed to identify the significant independent prognostic factors. The association between clinicopathological variables and PLA2G16 expression was evaluated by Pearson’s chi-squared test (χ2). Statistical analysis were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics and PLA2G16 expression in breast cancer specimens
A total of 200 specimens were included initially in our study, however, 17 cases were lost during IHC staining process. Therefore, the remaining 183 cases were included in the subsequent analysis. The clinicopathological characteristics of these patients are summarized in Table 1. The median age of the patients was 50.44 years (range, 26–84 years) and the median follow-up time was 94.38 months. Of these 183 specimens, 87 cases (47.5%) showed high expression of PLA2G16 by immunohistochemical staining (representative images; Figure 1). The relationships between PLA2G16 expression and various clinical variables were further investigated. The result indicated that PLA2G16 expression was associated with ER status (P=0.054), and patients with higher expression of PLA2G16 were less likely to be ER negative (Table 1). High expression of PLA2G16 exhibited a borderline correlation with smaller tumor size (P=0.066). Subsequently, we analysed the association between tumor size and PLA2G16 expression in the subgroups which were classified by the status of ER and HER2. However, there were no significant associations between them in any subgroups (Supplementary Table S1).
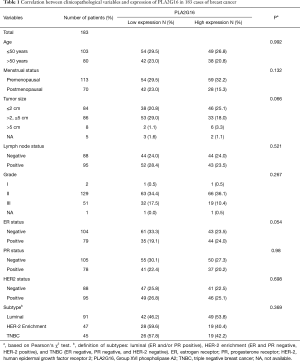
Full table
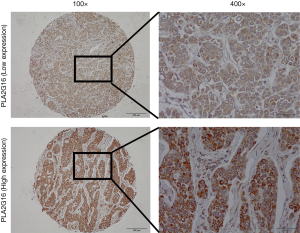
Full table
High PLA2G16 expression is associated with improved disease-free survival in breast cancer patients
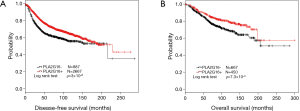
The correlation between clinicopathological variables and DFS was evaluated by univariate and multivariate analysis. As shown in Table 2, PLA2G16 was significantly associated with prolonged DFS in breast cancer patients. Furthermore, we used Kaplan-Meier analysis to evaluate the prognostic value of PLA2G16 for DFS and OS in all patients. As shown in Figure 2, PLA2G16 overexpression was significantly associated with prolonged DFS (P=0.032; Figure 2A) and trending associated with improved OS (P=0.166; Figure 2B). Moreover, consistent results between PLA2G16 expression and OS were obtained in several subgroups (Supplementary Figure S1). However, the analysis from the Kaplan-Meier Plotter database showed that the overexpression of PLA2G16 was correlated with both improved DFS (P=3×10-8; Figure 3A) and OS (P=7.3×10-4; Figure 3B). Considering that the peak of recurrence time is 7–10 years for luminal subtype breast cancer (16) and the 94.38-month median follow-up time of our cohort, we speculated that the recruitment of more patients with longer follow-up time may help to determine the association of PLA2G16 overexpression and OS of breast cancer patients in the future.

Full table
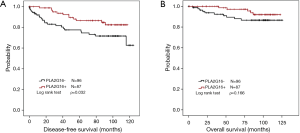

PLA2G16 is an independent prognostic factor in HER2-positive breast cancer
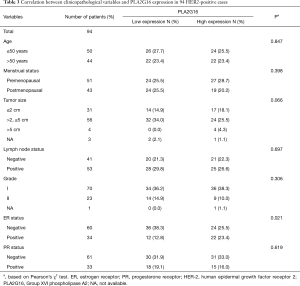
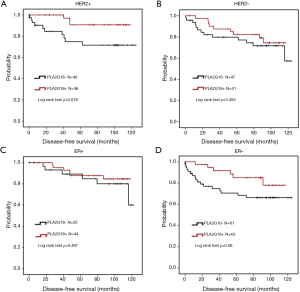
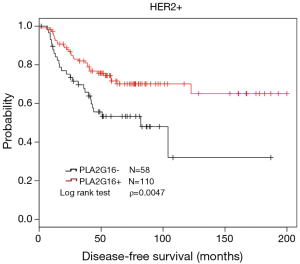
To further investigate the role of PLA2G16 expression in breast cancer with different receptor status, we conducted the analyses in patients with different ER and HER2 status. Interestingly, in the 95 cases with positive HER2 status, we found that PLA2G16 expression was correlated with ER status (P=0.021; Table 3). More importantly, there was a statistical association between elevated PLA2G16 expression and prolonged DFS in this subgroup (P=0.026; Table 4 and P=0.018; Figure 4A). PLA2G16 was found to be an independent prognostic indicator only in HER2-positive breast cancer [hazard ratio (HR) =0.151; 95% confidence interval (CI) =0.034–0.672; P=0.013; Figure 4, Figure S1 and Table 4], which is consistent with the Kaplan-Meier Plotter database (Figure 5). Notably, high PLA2G16 expression had a borderline correlation with better survival in the ER-negative breast cancer patients (Figure 4D).
Full table

Full table
Discussion
In this study, we demonstrated that overexpression of PLA2G16 is correlated with better DFS in breast cancer patients, which is supported by KM-Plotter database and consistent with that in ovarian carcinoma (5). PLA2G16 inhibits the growth of ovarian carcinoma (5), cervical (17) and testicular carcinoma cell (10). It was also reported that PLA2G16 suppresses invasion in testicular carcinoma (18) and induces apoptosis in ovarian carcinoma (7). However, PLA2G16 is also labeled as an oncogene in several other cancers. PLA2G16 was found to increase proliferation of non-small cell lung cancer (15) and promote metastasis and drug resistance in osteosarcoma (19) and its overexpression was associated with poor prognosis in both of them. Therefore, PLA2G16 may be a tumor type specific biomarker, the role of PLA2G16 in different types of tumors may be related with the status of receptors or hormones as indicated in this study.
In further study, we classified the specimens into several subgroups based on the ER and HER2 status. Interestingly, we found that PLA2G16 overexpression was correlated with better prognosis in the HER2-positive specimens, which supported by the Kaplan-Meier Plotter database (Figure 5). As we all know, approximately 20–30% of primary human breast cancer to be HER2 positive, due to overexpression and/or gene amplification of HER2 gene. HER2 overexpression induces cell growth, proliferation and survival via stimulated RAS/MAPK and PI3K signal pathways (20,21). Trastuzumab is considered to be a primary treatment for HER2 positive breast cancer patients. However, alternative mechanism which can activate RAS/MAPK signal pathway may lead to trastuzumab resistance in these patients (5). PLA2G16 is first identified as a tumor suppressor to inhibit HRAS induced transformation, cell proliferation, colony formation, and promote apoptosis (8,9,22,23). PLA2G16 exerts its anti-RAS activity through its PLA/AT activity to decrease the steady state levels of H-RAS palmitoylation in cervical cancer cells (17) and the suppression of RAS by PLA2G16 could be eliminated by AACOCF3 and MAFP (6,17). In ovarian carcinoma, PLA2G16 binds to p65 to induce apoptosis and its overexpression correlates with better prognosis (7). Taken together, we speculated that PLA2G16 may play a role in inhibiting cell growth or inducing apoptosis in breast cancer. It may also antagonize the resistance of trastuzumab by inhibiting RAS in HER2-positive breast cancer cells.
Acknowledgements
Funding: This study was supported by the Natural Science Foundation of Liaoning Province (Grant No. 20150315) and the Scientific Research General Project of the Liaoning Provincial Department of Education (Grant No. L2014348).
This study was also supported by a grant from the Ministry of Science and Technology of China (MOST2016YFC0900300), by the National Natural Science Foundation of China (81672601) and by the Shanghai Committee of Science and Technology Funds (15410724000 to X. Hu). The funders had no role in the study design, collection and analysis of the data, decision to publish, or manuscript preparation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Review Board of FUSCC (No. 050432-4-1212B), and written informed consent was obtained from all patients.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Perou CM, Jeffrey SS, van de Rijn M, et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci U S A 1999;96:9212-7. [Crossref] [PubMed]
- Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287-312. [Crossref] [PubMed]
- Perez EA. Treatment strategies for advanced hormone receptor-positive and human epidermal growth factor 2-negative breast cancer: the role of treatment order. Drug Resist Updat 2016;24:13-22. [Crossref] [PubMed]
- Gala K, Chandarlapaty S. Molecular pathways: HER3 targeted therapy. Clin Cancer Res 2014;20:1410-6. [Crossref] [PubMed]
- Duncan RE, Sarkadi-Nagy E, Jaworski K, et al. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA). J Biol Chem 2008;283:25428-36. [Crossref] [PubMed]
- Nazarenko I, Schäfer R, Sers C. Mechanisms of the HRSL3 tumor suppressor function in ovarian carcinoma cells. J Cell Sci 2007;120:1393-404. [Crossref] [PubMed]
- Sers C, Emmenegger U, Husmann K, et al. Growth-inhibitory activity and downregulation of the class II tumor-suppressor gene H-rev107 in tumor cell lines and experimental tumors. J Cell Biol 1997;136:935-44. [Crossref] [PubMed]
- Husmann K, Sers C, Fietze E, et al. Transcriptional and translational downregulation of H-REV107, a class II tumour suppressor gene located on human chromosome 11q11-12. Oncogene 1998;17:1305-12. [Crossref] [PubMed]
- Siegrist S, Féral C, Chami M, et al. hH-Rev107, a class II tumor suppressor gene, is expressed by post-meiotic testicular germ cells and CIS cells but not by human testicular germ cell tumors. Oncogene 2001;20:5155-63. [Crossref] [PubMed]
- Schaefer R, Iyer J, Iten E, et al. Partial reversion of the transformed phenotype in HRAS-transfected tumorigenic cells by transfer of a human gene. Proc Natl Acad Sci U S A 1988;85:1590-4. [Crossref] [PubMed]
- Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 2003;3:582-91. [Crossref] [PubMed]
- Nomura DK, Long JZ, Niessen S, et al. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010;140:49-61. [Crossref] [PubMed]
- Liang S, Ren Z, Han X, et al. PLA2G16 Expression in Human Osteosarcoma Is Associated with Pulmonary Metastasis and Poor Prognosis. PLoS One 2015;10:e0127236. [Crossref] [PubMed]
- Nazarenko I, Kristiansen G, Fonfara S, et al. H-REV107-1 stimulates growth in non-small cell lung carcinomas via the activation of mitogenic signaling. Am J Pathol 2006;169:1427-39. [Crossref] [PubMed]
- Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 2010;7:e1000279. [Crossref] [PubMed]
- Wang CH, Shyu RY, Wu CC, et al. Phospholipase A/Acyltransferase enzyme activity of H-rev107 inhibits the H-RAS signaling pathway. J Biomed Sci 2014;21:36. [Crossref] [PubMed]
- Shyu RY, Wu CC, Wang CH, et al. H-rev107 regulates prostaglandin D2 synthase-mediated suppression of cellular invasion in testicular cancer cells. J Biomed Sci 2013;20:30. [Crossref] [PubMed]
- Li L, Liang S, Wasylishen AR, et al. PLA2G16 promotes osteosarcoma metastasis and drug resistance via the MAPK pathway. Oncotarget 2016;7:18021-35. [PubMed]
- Castaneda CA, Cortes-Funes H, Gomez HL, et al. The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer Metastasis Rev 2010;29:751-9. [Crossref] [PubMed]
- Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 2014;13:140-56. [Crossref] [PubMed]
- Sers C, Husmann K, Nazarenko I, et al. The class II tumour suppressor gene H-REV107-1 is a target of interferon-regulatory factor-1 and is involved in IFNgamma-induced cell death in human ovarian carcinoma cells. Oncogene 2002;21:2829-39. [Crossref] [PubMed]
- Hajnal A, Klemenz R, Schäfer R. Subtraction cloning of H-rev107, a gene specifically expressed in H-ras resistant fibroblasts. Oncogene 1994;9:479-90. [PubMed]


