Protective effect of propofol preconditioning on ischemia-reperfusion injury in human hepatocyte
Introduction
Ischemia can induce a series of biochemical reactions, resulting in cell dysfunction, even cell death (1). Besides, it contributes to the pathophysiology of many conditions, such as peripheral vascular insufficiency, myocardial infarction, hypovolemic shock and stroke (2). Blood reperfusion after ischemia is the main measure to restore cell function. However, in some cases, reperfusion may aggravate the tissue damage, because severe metabolic disorders may appear due to the infusion of toxic metabolites and various inflammatory mediators which are formed during ischemia in the systemic circulation (3). Ischemia-reperfusion (I/R) injury in the liver is commonly encountered in some clinical settings, such as trauma, electric liver resection and liver transplantation (4). Currently, many strategies have been proposed to reduce the incidence of I/R injury in the liver, however, the risk has not been eliminated.
The mechanisms underlying I/R injury in liver are highly complex (5). Recently, more and more evidences suggest that excessive reactive oxygen species during the initial phase of reperfusion acts as a signaling molecule inducing the release of endogenous damage-associated molecular patterns, which are responsible for the damage of liver (6,7). Specially, propofol is an anesthesia induction drug with antioxidant property, the protective effects of which have been described in models of I/R injury in several organs (8,9). For instance, it has been reported to dose-dependently attenuate myocardial I/R injury in patients (10). A recent study shows that propofol attenuates brain trauma induced cerebral injury through inhibiting NADPH oxidase activation (11). Additionally, Pei et al. (12) demonstrated that propofol could protect I/R-induced lung injury by improving activity of oxygen free radical and restoring dynamic balance of nitric oxide/endothelin-1 (NO/ET-1). However, at present, there is hardly any study about the therapeutic role and mechanism of propofol in I/R-induced liver injury.
In the present study, we established the rat models and cell models of liver I/R injury and investigated the alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA) and adenosine triphosphate (ATP) levels in liver tissues and hepatocytes. Besides, we investigated the cell viability and apoptosis of hepatocytes. Furthermore, the expressions of apoptosis-related proteins mammalian B cell lymphoma-2 (Bcl-2) and caspase-3 in hepatocytes were determined to further study the molecular mechanisms. Our study aimed to explore the role of propofol in liver I/R injury.
Methods
Animals and tissues
All experimental procedures were in accordance with the protocols approved by the Ethics Committee for Animal Experimentation of the Shandong Provincial Hospital Affiliated to Shandong University. A total of 16 Wistar rats, weighing 200–220 g, were obtained from the Animal Center, Sichuan Academy of Medical Sciences (Sichuan, China). These rats were kept in animal house with free access to standard pellet diet and tap water under a 12/12 h light-dark cycle condition before the experiment. The rats were randomly designated into control group, sham group, I/R group, and propofol group (four rats per group). The gender distribution and weight had no statistical difference among different groups (P>0.05).
The rats in sham, I/R, and propofol groups were fasted for 12 h (fed by water freely) before being anesthetized with intraperitoneal injection of 25 mg/kg pentobarbital. Then the rats were subjected to median laparotomy under sterile conditions in the supine position. The hepatic artery, portal vein and ballast ductus were made visible, and then the abdomen of the rats were closed without executing any further procedures in sham group. In I/R group, the hepatic pedicle was identified and the hepatic artery, portal vein and bile ducts were occluded with an atraumatic vascular clamp for hepatic ischemia, which lasted for 90 min. Following 90 min of ischemia, the clamp was opened and the liver was reperfused for 6 h. For propofol group, 20 µmol/L propofol was intraperitoneally injected 2 h before surgery and the other steps were the same with that of I/R group.
For all rats, 2 mL blood samples were taken from their inferior vena cava before being killed, and the serum samples were separated by centrifugation at 1,500 rpm for 10 min. The liver tissue samples were taken and frozen with liquid nitrogen for biochemical determination.
Human hepatocyte culture
Human hepatocyte HL7702 was used in the study, which was purchased from Chinese Academy of Sciences Shanghai Institutes for Biological Sciences Cell resource center (Shanghai, China). The cells (5×105 cells/mL) were cultured in the culture flask with high sugar Dulbecco Modified Eagle Medium (DMEM) culture medium supplemented with 10% fetal bovine serum (FBS) and 22.5% F12 in a humidified atmosphere with 5% CO2 at 37 °C. When 80–90% of the bottom of flask was covered with cells, 1 mL trypsin-EDTA solution containing 0.25% trypsin and 0.02% EDTA was added to the culture flask for passage.
Hepatocyte I/R injury model
HL7702 hepatocytes were divided into six groups: control group, I/R group and propofol (5, 10, 20 and 40 µmol/L) groups. Hepatocytes were cultured in 96-well plates at a density of 2×104 cells/well (each group with 6 repeats) in a CO2 incubator with 5% CO2 at 37 °C. Two 96-well plates were used in this study. One was used for control group, and the other was used for the mentioned five groups.
One hour before I/R, hepatocytes in propofol groups were preconditioned with propofol at concentrations of 5, 10, 20 or 40 µmol/L. After propofol preconditioning, the hepatocytes in control group were cultured with complete medium for 8 h in the CO2 incubator. The hepatocytes in propofol groups and I/R group were cultured with 100 µL sugar-free DMEM medium in an anaerobic culture box which was put in the incubator. After 8 h of incubation, the mediums of six groups were all replaced with complete medium and were cultured in normal condition (37 °C and 5% CO2). The supernatant of cell culture was collected for biochemical determination.
Biochemical analysis
The ALT and AST levels of the obtained serum samples and cell culture supernatant were measured by the Beckman delta CX7 autoanalyzer. The content of MDA and ATP in liver tissues and cell culture supernatant were respectively measured by thiobarbituric acid (TBA) and bioluminescence methods according to the manufacturer’s instructions of reagent kits that were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). The liver tissues were firstly homogenized and centrifuged at 15,000 rpm for 10 min at 4 °C, the supernatant was used for further assays. For MDA measurement, 100 µL liver tissue supernatant or cell culture supernatant, 100 µL standard solution, 100 µL ethyl alcohol were respectively transferred to tubes as testing (control) tube, standard tube and blank testing tube. Then, reagent 1, 2 and 3 were added to each tube while the reagent 3 was replaced by 50% acetic acid in control tube. After shaking vigorously, the tubes were heated at 95 °C for 40 min. Finally, the mixture was cooled, centrifuged at 3,500 rpm for 10 min and measured at 532 nm. The content of total protein was determined by BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). The level of MDA was expressed as nanomoles or micromoles per milligram protein. For ATP measurement, 30 µL liver tissue supernatant or cell culture supernatant, 30 µL standard solution were respectively transferred to tubes as testing (control) tube, standard (blank) tube. Then, reagent 1, 2 were added to each tube. Subsequently, 30 µL reagent 3 and double distilled water were respectively added to standard (test) tube and blank (control) tube. After mixing and incubation at 37 °C for 30 min, reagent 4 was added to each tube. Following mixing, centrifugation at 4,000 rpm for 10 min, 300 µL supernatant was mixed with reagent 5 and restored at room temperature for 2 min. Finally, the reagent 6 was added to the mixture, restored at room temperature for 5 min and measured at 636 nm. The level of ATP was expressed as nanomoles or micromoles per milligram protein.
Cell viability assay
HL7702 hepatocytes of three groups (control, I/R, and 20 µmol/L propofol) were cultured in the 96-well plate at a density of 2×105 cells/mL (200 µL per well) for 24, 48 and 72 h. Each group had five repeats. Then 20 µL 5 g/L 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to each well. After 4 h incubation, 150 µL dimethyl sulfoxide (DMSO) was added to each well. The optical density (OD) was measured at 570 nm. The experiment was repeated for three times.
Apoptosis assay
HL7702 hepatocytes of three groups, i.e., control, I/R, and 20 µmol/L propofol, were respectively seeded into the culture dish at a density of 3×105 cells/mL. Then 5 µL annexin V-FITC and 5 µL proprium iodide (PI) were added according to the manufacturer’s instructions. Cells were analyzed with a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA). The results were analyzed using Cell FIT Cell Cycle Analysis Version 2.01.2 (BD Biosciences).
Western blot analysis
HL7702 hepatocytes were lysed in RIPA (Pirece Co., Shanghai, China). The total protein concentration was determined with BCA method. Protein samples were separated on a 10–12% SDS-PAGE and then blotted onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA). After that, the membranes were incubated for 1 h at room temperature and then probed with primary antibodies overnight at 4 °C. The primary antibodies included polyclonal rabbit antibodies to human caspase-3 (Santa Cruz, Santa Cruz, CA, USA), and monoclonal antibodies to human Bcl-2 (Santa Cruz) at a dilution of 1:500. Then membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (1:1,000). GAPDH was used as internal control. The protein bands were then washed and developed by enhanced chemiluminescence.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). The data were presented as mean ± SD. All collected data were firstly tested for the normal distribution using one-sample K-S test. Measurement data were tested by student t-test (for two groups) or one way analysis of variance (ANOVA, for more than three groups). P<0.05 was considered statistically significant.
Results
Effect of propofol on ALT, AST, MDA and ATP levels in rat liver tissue
When comparing with control group, the ALT, AST and MDA levels in I/R group were statistically higher (P=0.0021, P=0.0051 or P=0.0073), while ATP level in I/R group was significantly lower (P=0.0018). Similarly, the ALT, AST and MDA levels in I/R group were markedly increased (P=0.0017, P=0.0052 or P=0.0076), while ATP level in I/R group was remarkably decreased (P=0.0020) in comparison with sham group. However, when the rats were preprocessed with propofol, the ALT, AST and MDA levels decreased significantly (P=0.0080, P =0.0073 or P=0.0091), and the ATP level increased significantly compared with that of I/R group (P=0.0037). These findings are illustrated in Figure 1A-D.
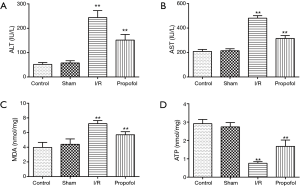
Effect of propofol on ALT, AST, MDA and ATP levels in human hepatocyte
The effects of propofol preprocessing on ALT, AST, MDA and ATP levels in human hepatocyte were shown in Figure 2. As shown in Figure 2A-C, the ALT, AST and MDA levels of human hepatocytes in I/R group were significantly higher than that in control group (P=0.00055, P=0.00085 or P=0.00025), while the ATP level in I/R group was significantly lower than that in control group (Figure 2D, P=0.00035). When compared to I/R group, the ALT, AST and MDA levels in propofol group (5, 10, 20 or 40 µmol/L, respectively) were significantly lower (P=0.9800, P=0.0476, P=0.0276 or P=0.0133 for ALT; P=0.0482, P=0.0447, P=0.0091 or P=0.0073 for AST; P=0.0481, P=0.0090, P=0.0060 or P=0.0055 for MDA), and the ATP level was significantly higher in propofol group (P=0.0493, P=0.0035, P=0.0021 or P=0.0018). Importantly, the effect of propofol on ALT, AST, MDA and ATP levels showed a concentration-dependent manner.
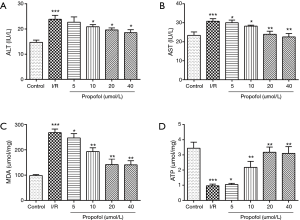
Effect of propofol on cell viability
MTT assay was performed to investigate the effect of propofol on hepatocyte viability. The results showed that compared with the control group, the cell viability declined significantly in I/R group at 24 h (P=0.0451), 48 h (P=0.0041) and 72 h (P=0.0037). However, when hepatocytes were preprocessed with 20 µmol/L propofol, the cell viabilities at 24, 48 and 72 h increased significantly (P=0.0455, P=0.0481 or P=0.0061) (Figure 3).
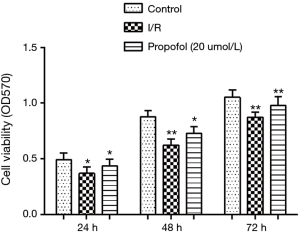
Effect of propofol on cell apoptosis
Significant differences in percentages of apoptotic cells were determined. In detail, the percentage of apoptotic cells was markedly enhanced in I/R group compared with that in control group (P=0.0110), while the percentage of apoptotic cells was decreased by preprocessing of 20 µmol/L propofol, resulting in non-significant difference compared with control group (P=0.0832). The result was shown in Figure 4A.

The expressions of apoptosis-related proteins including anti-apoptotic protein Bcl-2 and pro-apoptotic protein caspase-3 were determined by western blot analysis (Figure 4B). The result showed that the expression of Bcl-2 in I/R group was significantly lower than that in control group and the decrease was obviously increased by propofol preconditioning. On the other hand, the expression of caspase-3 was enhanced by I/R injury and reduced by propofol preconditioning.
Discussion
Reactive oxygen species produces during reperfusion is generally considered to play a key role in I/R injury by direct attack on a vast array of cellular molecules (13). Propofol is an commonly used intravenous anesthesia drug with the chemical component of 2,6-diisopropyl cresol which has strong anti-oxygenation effect. Propofol has been employed in clinical treatment of I/R in recent years (14). In the present study, biochemical investigations showed that the ALT, AST and MDA levels decreased significantly, and the ATP level increased significantly in propofol group compared with that in I/R group. Additionally, the cell viability in propofol group was higher than that in I/R group, while the percentage of apoptotic cells in propofol group was less than that in I/R group.
Hepatic I/R injury is a pathophysiological event after liver surgery or transplantation (15). The effective approach to reduce liver damage after hepatic I/R is lacking. Propofol is a widely used intravenous anesthetic for surgical procedures. A previous clinical study reported that propofol might lack protective properties on cardiac mechanical dysfunction and possess limitations on ultrastructural abnormality in the myocyte exposed to I/R, leading to worse outcomes compared with volatile anaesthetic agents on liver injury after partial hepatectomy in cirrhotic patients (16). However, a more recent study has reported that propofol exerts protective effect on liver damages in patients after partial hepatectomy (17). Considering the controversial results, the present study was aiming to identify whether propofol preconditioning is protective for I/R induced liver injury in both animal and cell levels.
ALT and AST indicate the concentration of hepatic intracellular enzymes that have leaked to the circulation, which are the markers for hepatocellular injury (18,19). Significantly elevated levels of ALT and AST often suggest the existence of some medical problems such as viral hepatitis, liver damage, and bile duct problems (20). Tuncer et al. (21) reported that hepatocyte necrosis and polymorphonuclear cell infiltration in I/R liver tissues were accompanied with an increase in ALT and AST levels. Additionally, Crockett et al. (22) have also determined elevated ALT levels, observed hepatocellular necrosis and neutrophil infiltration were existed in I/R liver tissues. Similarly, in our study, significantly increased ALT and AST levels as well as apoptotic cells were found in I/R group. However, after propofol preconditioning, the ALT and AST levels and apoptotic cells declined significantly, indicating that propofol exerted protective effects against I/R-induced liver injury.
Evidence has implicated that free radicals is one of major contributors to ischemic tissue injury (23). MDA is an important free radical, which is widely used as an indicator of free radical-mediated lipid peroxidation injury (24). It has been found elevated in various diseases (25). In this study, the MDA levels increased significantly following liver I/R. However, propofol reversed the increase of MDA levels to a considerable extent, thereby confirming their antioxidant role in liver I/R. ATP is a nucleoside triphosphate used in cells as a coenzyme (26), which plays a critical role in I/R preconditioning in myocardium and endothelium (27,28). Importantly, propofol has been reported to affect by activating ATP-sensitive potassium channels in I/R-induced cell apoptosis in an renal I/R model (29). Therefore, the increased level of ATP after propofol preconditioning in this study may suggest the protection of propofol against liver I/R injury.
Apoptosis is a complex biological process that enables organisms to kill and remove unwanted cells during their development (30,31). Study has found that I/R injury can activate various cell death signaling programs including apoptosis, necrosis, and autophagy-related cell death (32). For example, Chaitanya et al. (33) suggested that ischemia contributed to the apoptosis of neurons. Importantly, apoptosis is recognized as an important sequela of hepatic I/R injury (34). Consistent with the findings above, the percentage of apoptotic cells in I/R group was significantly higher than that in control group, besides, the cell viability in I/R group was significantly lower compared with that in control group. Specially, after propofol preconditioning, the percentage of apoptotic cells decreased, at the same time, the cell viability increased significantly.
Ischemic cell death is often due to the involvement of some apoptosis-related proteins, such as proteins in caspase and Bcl-2 families (33,35,36). These proteins are key mediators in the induction of apoptosis in different cells (37). Caspase-3 is a member of the caspase family of aspartate-specific cysteine proteases, playing an important role in apoptotic program (38). Activation of caspase-3 leads to DNA fragmentation (39). Caspase-3 gene expression have been found increased in the hippocampus after global ischemia (40). Bcl-2, an anti-apoptotic protein, can inhibit cytochrome c from translocating to the cytosol which is a critical step in the apoptotic process (41). Many studies have demonstrated that overexpression of Bcl-2 reduces ischemic injury (42,43). In our study, the expression of caspase-3 decreased and the expression of Bcl-2 increased after propofol preconditioning, which indicated that propofol played an important role in inhibiting the apoptosis of hepatocytes in I/R injury.
The blood pressure during surgery might be an important mediator involving in the effect of propofol on I/R injury. In our study, the rats were hemodynamically stable and the potential effect of hypotension was avoided. In addition, although we found propofol protected I/R injury of liver by enhanced cell viability and inhibited apoptosis, the underlying mechanism remains unclear. Propofol has been suggested to alleviate liver oxidative stress through activating nuclear factor-E2-related factor 2 (Nrf2) pathway (44). Meantime, propofol also has been reported to ameliorate I/R injury of rat liver by inhibition of nuclear factor-κB (NF-κB) expression (45). Another study reported that propofol could preserve liver from I/R injury through glycogen synthase kinase 3β (GSK-3β), which is known to affect oxidative stress-induced apoptosis (46). Therefore, we hypothesized that the protective effect of propofol might be associated with Nrf2 and NF-κB pathways as well as GSK-3β. The concrete mechanism need to be further studied.
Conclusions
In conclusion, our results indicated the protective effects of propofol on I/R-induced liver injury, which may be achieved by enhanced cell viability and inhibited apoptosis. Therefore, propofol preconditioning may be an effective strategy for protecting the liver from I/R injury.
Acknowledgements
Funding: This study was supported by the Natural Science Foundation of Shandong Province (No. ZR2012HM092) and the National Natural Science Foundation of China (No. 30872433).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethics Statement: The study was approved by the Ethics Committee for Animal Experimentation of the Shandong Provincial Hospital Affiliated to Shandong University (No. IACUC-11-016).
References
- Baykara B, Tekmen I, Pekcetin C, et al. The protective effects of carnosine and melatonin in ischemia-reperfusion injury in the rat liver. Acta Histochemica 2009;111:42-51. [Crossref] [PubMed]
- Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001;94:1133. [Crossref] [PubMed]
- Kaszaki J, Wolfárd A, Szalay L, et al. Pathophysiology of Ischemia-Reperfusion Injury. Transplant Proc 2006;38:826-8. [Crossref] [PubMed]
- Cardinal J, Pan P, Tsung A. Protective role of cisplatin in ischemic liver injury through induction of autophagy. Autophagy 2009;5:1211-2. [Crossref] [PubMed]
- Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg 2003;10:189-94. [Crossref] [PubMed]
- Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 2005;201:1135-43. [Crossref] [PubMed]
- Gan X, Xing D, Su G, et al. Propofol Attenuates Small Intestinal Ischemia Reperfusion Injury through Inhibiting NADPH Oxidase Mediated Mast Cell Activation. Oxid Med Cell Longev 2015;2015:167014.
- Kobayashi I, Kokita N, Namiki A. Propofol attenuates ischaemia-reperfusion injury in the rat heart in vivo. Eur J Anaesthesiol 2008;25:144-51. [Crossref] [PubMed]
- Azeredo MA, Azeredo LA, Eleuthério EC, et al. Propofol and N-Acetylcysteine attenuate oxidative stress induced by intestinal ischemia/reperfusion in rats: protein carbonyl detection by immunoblotting. Acta Cirurgica Brasileira 2008;23:425-8. [Crossref] [PubMed]
- Xia Z, Huang Z, Ansley DM. Large-dose propofol during cardiopulmonary bypass decreases biochemical markers of myocardial injury in coronary surgery patients: a comparison with isoflurane. Anesth Analg 2006;103:527-32. [Crossref] [PubMed]
- Luo T, Wu J, Kabadi SV, et al. Propofol limits microglial activation after experimental brain trauma through inhibition of nicotinamide adenine dinucleotide phosphate oxidase. Anesthesiology 2013;119:1370-88. [Crossref] [PubMed]
- Yang P, Yang N, Zhang X, et al. The significance and mechanism of propofol on treatment of ischemia reperfusion induced lung injury in rats. Cell Biochem Biophys 2014;70:1527-32. [Crossref] [PubMed]
- Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med 2010;48:1121-32. [Crossref] [PubMed]
- Huerta L, Rancan L, Simón C, et al. Ischaemic preconditioning prevents the liver inflammatory response to lung ischaemia/reperfusion in a swine lung autotransplant model. Eur J Cardiothorac Surg 2013;43:1194-201. [Crossref] [PubMed]
- Guan LY, Fu PY, Li PD, et al. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg 2014;6:122-8. [Crossref] [PubMed]
- Yang LQ, Tao KM, Cheung CW, et al. The effect of isoflurane or propofol anaesthesia on liver injury after partial hepatectomy in cirrhotic patients. Anaesthesia 2010;65:1094-100. [Crossref]
- Laviolle B, Basquin C, Aguillon D, et al. Effect of an anesthesia with propofol compared with desflurane on free radical production and liver function after partial hepatectomy. Fundam Clin Pharmacol 2012;26:735-42. [Crossref] [PubMed]
- Ni H, Soe HH, Htet A. Determinants of abnormal liver function tests in diabetes patients in Myanmar. Int J Diab Res 2012;1:36-41. [Crossref]
- Lee TH, Kim W, Benson JT, et al. Serum aminotransferase activity and mortality risk in a United States community. Hepatology 2008;47:880-7. [Crossref] [PubMed]
- Hyder MA, Hasan M, Mohieldein AH. Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Eur J Exp Biol 2013;3:280-4.
- Tuncer MC, Ozturk H, Buyukbayram H. Interaction of L-arginine-methyl ester and Sonic hedgehog in liver ischemia-reperfusion injury in the rats. World J Gastroenterol 2007;13:3841-6. [Crossref] [PubMed]
- Crockett ET, Galligan JJ, Uhal BD, et al. Protection of early phase hepatic ischemia-reperfusion injury by cholinergic agonists. BMC Clin Pathol 2006;6:3. [Crossref] [PubMed]
- Hosseinzadeh H, Parvardeh S, Asl MN, et al. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine 2007;14:621-7. [Crossref] [PubMed]
- Fukuda K, Asoh S, Ishikawa M, et al. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun 2007;361:670-4. [Crossref] [PubMed]
- Mateos R, Lecumberri E, Ramos S, et al. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress: Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J Chromatogr B Analyt Technol Biomed Life Sci 2005;827:76-82. [Crossref] [PubMed]
- Knowles JR. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem 1980;49:877-919. [Crossref] [PubMed]
- Costa AD, Pierre SV, Cohen MV, et al. cGMP signalling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res 2008;77:344-52. [Crossref] [PubMed]
- Testai L, Rapposelli S, Calderone V. Cardiac ATP-Sensitive Potassium Channels: A Potential Target for an Anti-Ischaemic Pharmacological Strategy. Cardiovasc Hematol Agents Med Chem 2007;5:79-90. [Crossref] [PubMed]
- Assad AR, Delou JO, Fonseca LM, et al. The Role of K-ATP Channels on Propofol Preconditioning in a Cellular Model of Renal Ischemia-Reperfusion. Anesth Analg 2009;109:1486-92. [Crossref] [PubMed]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995;267:1456. [Crossref] [PubMed]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell 2011;147:742-58. [Crossref] [PubMed]
- Kim SJ, Eum HA, Billiar TR, et al. Role of heme oxygenase 1 in TNF/TNF receptor-mediated apoptosis after hepatic ischemia/reperfusion in rats. Shock 2013;39:380-8. [Crossref] [PubMed]
- Chaitanya GV, Babu PP. Activation of calpain, cathepsin-b and caspase-3 during transient focal cerebral ischemia in rat model. Neurochem Res 2008;33:2178-86. [Crossref] [PubMed]
- Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis 2010;30:402-10. [Crossref] [PubMed]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 2004;116:205-19. [Crossref] [PubMed]
- Galluzzi L, Vitale I, Abrams J, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012;19:107-20. [Crossref] [PubMed]
- Narbutt J, Boncela J, Smolarczyk K, et al. Pathogenic activity of circulating anti-desmoglein-3 autoantibodies isolated from pemphigus vulgaris patients. Arch Med Sci 2012;8:347-56. [Crossref] [PubMed]
- Shabbir M, Syed DN, Lall RK, et al. Potent Anti-Proliferative, Pro-Apoptotic Activity of the Maytenus Royleanus Extract against Prostate Cancer Cells: Evidence in In-Vitro and In-Vivo Models. PloS One 2015;10:e0119859. [Crossref] [PubMed]
- Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997;91:479-89. [Crossref] [PubMed]
- Li J, Han B, Ma X, et al. The effects of propofol on hippocampal caspase-3 and Bcl-2 expression following forebrain ischemia–reperfusion in rats. Brain Res 2010;1356:11-23. [Crossref] [PubMed]
- Dumas TC, McLaughlin JR, Ho DY, et al. Gene therapies that enhance hippocampal neuron survival after an excitotoxic insult are not equivalent in their ability to maintain synaptic transmission. Exp Neurol 2000;166:180-9. [Crossref] [PubMed]
- Lawrence MS, Ho DY, Sun GH, et al. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J Neurosci 1996;16:486-96. [PubMed]
- Martinou JC, Dubois-Dauphin M, Staple JK, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 1994;13:1017-30. [Crossref] [PubMed]
- Ge M, Yao W, Wang Y, et al. Propofol alleviates liver oxidative stress via activating Nrf2 pathway. J Surg Res 2015;196:373-81. [Crossref] [PubMed]
- He J, Lu KZ, Tao GC. Propofol ameliorates rat liver ischemia-reperfusion injury possibly by inhibiting nuclear factor-kappaB expression. Nan Fang Yi Ke Da Xue Xue Bao 2008;28:1064-6. [PubMed]
- Zhao G, Ma H, Shen X, et al. Role of glycogen synthase kinase 3β in protective effect of propofol against hepatic ischemia-reperfusion injury. J Surg Res 2013;185:388-98. [Crossref] [PubMed]


