Nomogram prediction for the survival of the patients with small cell lung cancer
Introduction
Small cell lung cancer (SCLC) is a subtype of lung cancer with poor prognosis. It is estimated that nearly two million individuals are diagnosed as lung cancer every year, approximately 15% of which are SCLC (1). SCLC is characterized by a rapid doubling time and the propensity for early dissemination. Chemotherapy remains the first line therapy for SCLC. Despite the initial response to chemotherapy, most tumors ultimately would develop drug resistance which is associated with the unsatisfied prognosis. Only 10–15% of patients with limited disease (LD) are still alive 2 years after diagnosis, while the overall survival (OS) of patients with extensive disease (ED) is even shorter (2-4).
Tumor staging is commonly used to predict the prognosis of patients. The two most widely used staging systems for SCLC are Veterans Administration Lung Study Group (VALSG) system and the seventh edition of American Joint Committee on Cancer (7th AJCC) TNM classification. VALSG staging system stratifies the SCLC patients into LD and ED based on whether all tumors are within the scope of single tolerable radiotherapy field or not (5). Whilst, the 7th TNM classification stratifies the patients according to the extent of the primary tumor (T-stage), lymph node involvement (N-stage) and distant metastasis (M-stage) (6-8). However, the survival of the SCLC patients in the same stage varied dramatically, previous studies showed that various clinical factors of SCLC patients were predictive factors (9-12). Unfortunately, the predictive value of these factors remained unknown.
A nomogram is a graphical representation of a mathematical model involving several factors to predict a particular endpoint based on statistical methods (13). Through incorporating significant factors, nomograms could provide an estimated probability of an event, such as death or recurrence, which is tailored to the profile of individual patients. Nomograms have been considered as reliable tools to predict the clinical outcomes in many cancer types (14-16). The prediction of the nomogram has been proved to be more precise than the current staging systems in several types of cancer (15,16).
To predict the clinical outcome of SCLC patients more accurately, we therefore employed this strategy to investigate the prognostic value of various clinical variables, and establish a nomogram by analyzing the Chinese SCLC patients from the First Affiliated Hospital of Guangzhou Medical University (GMUFAH). We also validated the proposed nomogram in a separate cohort of Chinese SCLC patients.
Methods
Study cohort and data collection
This retrospective study was approved by the institutional Review Board of GMUFAH and Cancer Center of Guangzhou Medical University (GMUCC) (GMUFAH approval No: 2017-03; GMUCC approval No: P2017-001). In the primary cohort, 388 patients who were diagnosed as SCLC were constitutively enrolled from GMUFAH between January 2009 and December 2013. Only patients with SCLC as the initial diagnosis in the hospital were included. Patients who were previously received chemotherapy, radiotherapy or other anti-cancer treatment were excluded. And patients with incomplete data of eligible variables or follow-up data were also excluded.
In addition, 165 patients who were diagnosed as SCLC were also enrolled as a separate cohort from GMUCC between 2009 and 2013. After applying the exclusion criteria, a total of 80 SCLC patients were eventually included in this study for an external validation.
Clinical information retrieval
The patients’ clinicopathological information was retrieved from the electronic medical record system. We recorded the characteristics of all patients, including gender, age, smoking status, tumor size, underlying disease, disease stage, number of lesions, pathology subtype, pretreatment basic laboratory parameters and therapeutic strategies. The AJCC 7th TNM staging system and VALSG staging system were used to classify the patients. In all patients initial staging included CT scans of the thorax, upper abdomen and bone scintigraphy. The patients would undergo the brain MRI scan at initial diagnosis if the brain metastasis was highly suspected. Bronchoscopy or CT-guided biopsy was also used to tumor staging and sample the tumor for histopathology. Laboratory parameters were collected from hematologic tests which had been performed at initial diagnosis and prior to any anti-cancer treatment. These data included hemoglobin, white blood count (WBC), serum sodium, neutrophil-, lymphocyte- and platelets counts, blood albumin, gamma-glutamyltranspeptidase (GGT), fibrinogen (FIB), carcinoembryonic antigen (CEA), neuron specific enolase (NSE), cytokeratin 19 fragment (CYFRA21-1) and red cell distribution width (RDW).
Definition of NLR and PLR
The neutrophil-to-lymphocyte ratio (NLR) is defined as the absolute neutrophil granulocyte count divided by the absolute lymphocyte count, and the PLR was defined as the absolute platelet count divided by the absolute lymphocyte count (17).
Follow-up
The follow-up information was retrieved directly from the electronic medical record system or obtained by phone call. The last follow-up was obtained on May 31, 2015. The OS was calculated from the date of diagnosis to May 31, 2015 or death for any cause. Patients who did not die at the last follow-up were defined as alive. Patients who were lost to follow-up were defined as censored.
Data analysis
Continuous variables were presented as means ± SD or medians and ranges. Frequencies and proportions were calculated for categorical variables. Continuous variables were compared using the t-test or the non-parametric Mann-Whitney U test as indicated. Chi-square tests or the Fisher’s exact tests were adopted to compare between groups. The optimal cutoff values for NLR and PLR were determined using time-dependent receiver operating curve (ROC) analysis with survival ROC package (17). NLR and PLR cutoff points of 4.5 (11,12) and 258 (11) were used to stratify the patients, respectively.
In terms of survival analyses, Kaplan-Meier method with log-rank test was used to analyze the correlation between variables and OS. We used the Cox regression model to conduct the multiple factor analysis. The factors in multiple regression were selected using a backward step-down process, while the smallest Akaike’s information criterion (AIC) was used as a stopping rule. On the basis of the final model, a nomogram was built by R software with the rms package. To compare the performances between nomogram and other staging systems, we used rcorrp.cens package of Himsc.
Construction of nomogram
The independent covariates were selected using AIC. The nomogram was then constructed based on the results of multiple survival analysis according to the method as described previously. The scheme of nomogram was drawn using R package. The survival curves were depicted using Kaplan-Meier methods and were compared with log-rank test.
Validation and calibration of the nomogram
The nomogram was subjected to 1,000 bootstrap resamples for internal validation of the primary cohort (GMUFAH data) and external validation with the GMUCC cohort. The model performance was evaluated with respect to discrimination and calibration. Discrimination was quantified in the form of concordance index (C-index), which was similar to the area under the receiver-operating characteristics (ROC) curves (18). The C-index was calculated using survConcordance function in rms package in R software and used to compare the performance between the nomogram and the TNM or VALSG staging systems. Calibration was estimated by graphic representations of the relationships between observed outcome frequencies and predicted probabilities (calibration cures) for the groups of patients defined by quartiles (each quartile included at least 68 patients) (18). The overlap with reference line indicated perfect agreement on the model. We also compared the performance of our nomograms with those of the GMUCC.
All statistical analyses were performed using SPSS ver.19.0 software (SPSS Inc. Chicago, IL, USA) and R software ver.3.1.3 (http://www.r-project.org/). Two-tailed P values <0.05 were considered statistically significant.
Results
Patient’s characteristics
A total of 388 patients diagnosed as SCLC in GMUFAH between 2009 and 2013 were retrospectively enrolled in this study. The patients who were lost to follow up (n=95), or had received prior chemotherapy/radiotherapy (n=18) were excluded. Eventually, a total of 275 patients were included in this study after applying the exclusion criteria. For validation cohort, we enrolled 165 patients from GMUCC. And a total of 80 patients with SCLC were included in the validation cohort according to our inclusive and exclusive criteria. The detailed clinical pathologic characteristics of the patients in the primary cohort and validation cohort were listed in Table 1. In the primary cohort, the median age at diagnosis was 62 years (range, 33–86 years), majority of patients were smokers (179/275, 70%) and male (239/275, 87%), which were consistent with the previous reports (19). There were 235 events (deaths). The median OS was 12 months (range, 0–86 months), and the 1-, 2-year OS rates were 50% and 21%, respectively.
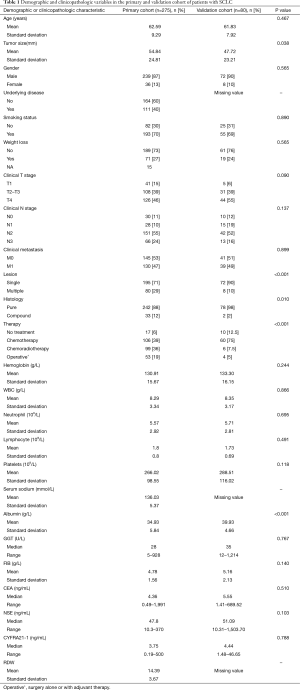
Full table
Independent prognostic factors in the primary cohort
We performed the univariate analysis to identify the clinical parameters which were significantly associated with OS of the patients. As shown in Table 2, age, tumor size, number of the lesions, type of histology, T stage, lymph nodes metastasis (N stage) and distant metastasis (M stage) were significantly associated with OS. A set of laboratory variables including NLR and PLR were also determined as significant factors that had significant impact on the survival. With respect to the factors associated with treatment, the patients who received surgery had better survival compared to those who did not. Whether surgery was an effective treatment in SCLC patients was still controversial. Some current studies reported by various institutes had put such topic back under debate, especially in the early stage disease (T1–T2N1). In our study, 37 patients with stage T1–2N0–1M0 were analyzed. Among them,22 patients underwent surgery, 15 patients received chemotherapy with or without radiotherapy. According to univariate analysis, patients who received surgery had improved 2-year survival rates compared with patients without operation. Although the sample size was insufficient, it indicated that surgery with or without adjuvant chemotherapy was associated with significantly improved OS (median OS 25.5 vs. 14 months; 2-year OS 66.0% vs. 20.0%, P<0.01). Similarly, chemotherapy with or without radiotherapy were also found to be associated with favorable outcomes.
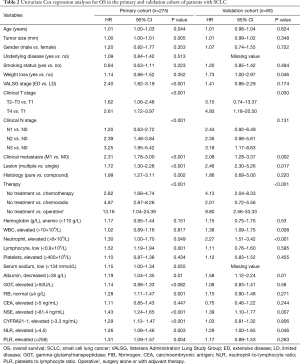
Full table
All the original factors entered into multiple Cox regression. In order to identify the independent covariates which were strikingly contributed to the prognosis, AIC was used to do variable selection. As shown in Table 3, seven important predictors were identified including age, N stage, M stage, histology, NSE, CYFRA21-1, and PLR.
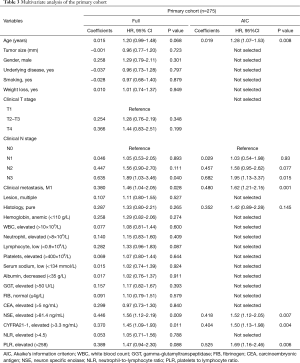
Full table
Nomogram model
All the significant factors identified by multiple factor analysis were used to establish the nomogram. As shown in Figure 1, the nomogram predicted 1- and 2-year OS that was constructed based on selected variables with hazard ratio. The nomogram revealed that N stage had the largest contribution to the prognosis, followed by PLR and M stage. The total score projected to the bottom scale indicates the probability of 1- and 2-year survival. The C-index calculated for the independent primary cohort was 0.68 (95% CI, 0.64–0.72), indicating excellent accuracy in predicting OS in this group of patients. Moreover, the results were internally validated using 1,000× bootstrap resampling (C-index =0.65).
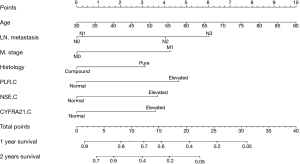
Comparison of predictive accuracy for OS between nomogram and existing staging system
We also compared the accuracy of prediction between this nomogram and the 7th TNM and VALSG staging system. As shown in Table 4, the performance of nomogram discrimination was 0.68 (95% CI, 0.64–0.72), which was superior to the 7th TNM classification (0.65; 95% CI, 0.62–0.69; P<0.01). Moreover, the C-index was higher in nomogram model than the VALSG (C-index, 0.68 vs. 0.66, P<0.01). The results suggested that this nomogram provided more accurate prediction in OS than existing staging systems both in the short and long term survival.

Full table
Validation of the predictive accuracy of the Nomogram for OS
The calibration plots presented an excellent agreement in the primary cohort between the nomogram prediction and actual observation for 1- and 2-year OS (Figure 2). We further performed external validation in an independent cohort of 80 patients with SCLC from GMUCC. As shown in Figure 3, the calibration curves showed great agreement between the nomogram prediction and actual observation for the 1-, 2-year OS. This result indicated that this nomogram was useful for predicting the survival of patients with SCLC.


Discussion
SCLC is a subtype of lung cancer associated with dismal prognosis. The 7th TNM classification and VALSG staging system are the most widely used models to predict the clinical outcome of SCLC currently. However, the prognosis of some patients at same stage in the conventional staging systems varies widely. A number of factors might contribute to such heterogeneity. Firstly, SCLC had a completely different biological behavior and pathological structure comparing to the NSCLC. In addition, recent progress in the cancer genome sequencing of SCLC unveiled that SCLC was a relative heterogeneous disease characterized by various genetic alterations such as mutations in TP53, RB1 and Notch, and copy number variants in chromosome 3p, JAK2, FGFR1, and MYC as well (20-22). Therefore, it was inappropriate that SCLC and NSCLC shared the same or similar staging system. On the other hand, SCLC patients were divided into only two categories in VALSG, which might be not conclusive (9-12).
Nomograms are a pictorial representation of a complex mathematical formula. Medical nomograms use biological and clinical variables to determine a statistical prognostic model that generates a probability of a clinical event, such as cancer recurrence or death, for a particular individual. For example, one patient’s tumor grade and age could be used to estimate his/her probability of cancer recurrence or death based on the nomogram. To better predict the OS of SCLC, in this study we have developed a novel nomogram by incorporating the clinically and laboratory relevant prognostic factors. In fact, the seven factors (including age, lymph node metastasis (N stage), distant metastasis (M stage), histology, NSE, PLR and CYFRA21-1) have been reported to be associated with the survival of SCLC in previous studies (19,23).
It is generally believed that most of the tumors are infiltrated by immune and inflammation cells, and inflammation is considered as one of the hallmarks of cancer (24). In this study, we found that the increased levels of NLR and PLR were associated with worse clinical outcome, which was consistent with the previous studies (25,26), although the role of inflammation cell in the tumor pathogenesis was not fully elucidated.
Additionally, it has been reported that the plasma level of FIB was elevated in malignancy or systemic inflammation diseases. Some studies showed that the level of FIB might be associated with tumor metastasis, stroma formation and angiogenesis (10). However, the level of FIB had little impact on the survival of SCLC in our study. It might attribute to the unique biological character of SCLC.
In recent years, many clinical trials have proven that surgery could extend the survival of LD-SCLC. The study of Shepherd et al. (27) showed that 5 years survival rate were nearly 40%, Yu’s (28) research reported operation combined with chemotherapy/radiotherapy was an effective method to treat stage I SCLC patients. Three- and 5-year survival rate were 78.9% and 64.9%, respective. In our study, we also found that surgery had the greatest contributions to the prognosis, which suggested that surgery should be recommended for some specific patients, especially the early stage patients (29).
This nomogram was shown to provide prediction of better accuracy for the OS of SCLC. We showed that this nomogram was superior to the existing staging systems with higher C-index and optimal agreement between prognostic prediction and actual observation. Furthermore, we validated its accuracy in an independent cohort of patients with SCLC, which also showed superiority to the conventional staging systems.
Limitations have to be admitted in this study. First, the primary cohort and the validation cohort were not large enough which might attribute to the low incidence of SCLC. Second, the SCLC patients in these two cohorts were identify and included from two different institutes. Furthermore, it was a retrospective study. Therefore, some tests or clinical data of patients were not uniform. Finally, the follow-up was performed via telephone which was not as detailed as the re-admission. Despite these limitations, the current study first developed the practical nomogram prediction for the survival of the patients with SCLC.
In conclusion, this proposed nomogram provided accurate prediction for the prognosis of the patients with SCLC. Further studies with large sample size are required to warrant its application in other ethnic patients with SCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the institutional Review Board of GMUFAH and Cancer Center of Guangzhou Medical University (GMUCC) (GMUFAH approval No: 2017-03; GMUCC approval No: P2017-001).
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Lebitasy MP, Hédelin G, Purohit A, et al. Progress in the management and outcome of small-cell lung cancer in a French region from 1981 to 1994. Br J Cancer 2001;85:808-15. [Crossref] [PubMed]
- Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease? Lung Cancer 2002;37:271-6. [Crossref] [PubMed]
- Jänne PA, Freidlin B, Saxman S, et al. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer 2002;95:1528-38. [Crossref] [PubMed]
- Shepherd FA, Ginsberg RJ, Haddad R, et al. Importance of clinical staging in limited small-cell lung cancer: a valuable system to separate prognostic subgroups. The University of Toronto Lung Oncology Group. J Clin Oncol 1993;11:1592-7. [Crossref] [PubMed]
- Lababede O, Meziane M, Rice T. Seventh edition of the cancer staging manual and stage grouping of lung cancer: quick reference chart and diagrams. Chest 2011;139:183-9.
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [Crossref] [PubMed]
- Zarogoulidis K, Latsios D, Porpodis K, et al. New dilemmas in small-cell lung cancer TNM clinical staging. Onco Targets Ther 2013;6:539-47. [Crossref] [PubMed]
- Zhou T, He X, Fang W, et al. Pretreatment Albumin/Globulin Ratio Predicts the Prognosis for Small-Cell Lung Cancer. Medicine (Baltimore) 2016;95:e3097. [Crossref] [PubMed]
- Takeuchi H, Ikeuchi S, Kitagawa Y, et al. Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol 2007;22:2222-7. [Crossref] [PubMed]
- Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo) 2015;70:524-30. [Crossref] [PubMed]
- Kang MH, Go SI, Song HN, et al. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer 2014;111:452-60. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012;30:3834-40. [Crossref] [PubMed]
- Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337-44. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Yip D, Harper PG. Predictive and prognostic factors in small cell lung cancer: current status. Lung Cancer 2000;28:173-85. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- Kahnert K, Kauffmann-Guerrero D, Huber RM. SCLC-State of the Art and What Does the Future Have in Store? Clin Lung Cancer 2016;17:325-33. [Crossref] [PubMed]
- Brueckl WM, Herbst L, Lechler A, et al. Predictive and prognostic factors in small cell lung carcinoma (SCLC)--analysis from routine clinical practice. Anticancer Res 2006;26:4825-32. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Li S, Xu X, Liang D, et al. Prognostic value of blood neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in patients with gastric cancer. Zhonghua Zhong Liu Za Zhi 2014;36:910-5. [PubMed]
- Shao N, Cai Q. High pretreatment neutrophil-lymphocyte ratio predicts recurrence and poor prognosis for combined small cell lung cancer. Clin Transl Oncol 2015;17:772-8. [Crossref] [PubMed]
- Shepherd FA. Surgery for limited stage small cell lung cancer: time to fish or cut bait. J Thorac Oncol 2010;5:147-9. [Crossref] [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [Crossref] [PubMed]
- Hamilton G, Rath B, Ulsperger E. A review of the role of surgery for small cell lung cancer and the potential prognostic value of enumeration of circulating tumor cells. Eur J Surg Oncol 2016;42:1296-302. [Crossref] [PubMed]


