Retrospective analysis of stereotactic ablative radiotherapy (SABR) for metastatic lung lesions (MLLs) in comparison with a contemporaneous cohort of primary lung lesions (PLLs)
Introduction
Based on conceptual theories of breast cancer growth and dissemination, Hellman and Weichselbaum inferred the existence of a clinically and biologically relevant group of oligometastatic patients, in whom aggressive local therapy may prolong survival and even be a curative treatment option (1). The incentive for using stereotactic ablative radiotherapy (SABR) for metastatic lung lesions (MLLs) came from favorable results of surgical removal of oligometastases in different types of solid tumors (2). High rates of survival in treated compared with untreated patients have also been demonstrated for lung metastasectomy (3). However, the temporal and locational burden of oligometastatic state remains blurred and diffuse and—until now—no clear guidelines have been defined for the selection of patients who would really benefit from local ablative treatment.
During the past decade, SABR emerged as a new standard of care for medically inoperable patients with early stage non-small-cell lung cancer. For oligometastatic diseases the benefit from SABR has been extrapolated from the experience with primary lung lesions (PLLs). However, patients with MLLs represent a clinically and biologically distinct population regarding the different clinical basic characteristics and the biology of the already disseminated cancer, therefore the efficacy and safety of SABR would not be expected to be identical to that of patients with PLLs. In this population, the progression-free survival (PFS) remains the crucial endpoint. There is some evidence that cancer metastases themselves may become the source of additional metastases (4). Patients with MLLs may gain benefit from a high local control (LC) of their oligometastases, if they are at a very low risk to develop further distant metastases (5). There have been a few studies that addressed the issue of comparison between MLLs and PLLs patients (5-8). In contrast to them, lung lesions of pulmonary origin, i.e., metastases from lung cancer were strictly excluded from the MLLs group. Also, the presented study provides a more holistic approach by analyzing a multitude of patient-, lesion- and treatment-related factors, which potentially determine the treatment outcome.
Methods
Patients
A positive statement of the local ethics committee (Ärztekammer des Saarlandes) was obtained prior to the data acquisition with the need for approval being waived due to use of anonymized data only. Between October 2011 and October 2014, we identified 80 lesions in 60 patients with lung tumors, who were consecutively treated with SABR after informed consent. All patients had previously been discussed by a multidisciplinary tumor board with a final consensus decision for lung SABR. Three patients were lost to follow-up and excluded, leaving a total of 28 lesions in 17 patients with lung metastases and 49 lesions in 40 patients with primary lung cancer which were retrospectively analyzed.
MLLs were defined as the presence of a new or an enlarging nodule or mass detected on routine chest imaging during the regular follow-up of a previously treated primary cancer (except for primary lung cancer, germ cell tumors and hematological malignancies). In 13 of 17 (76.5%) patients, the diagnosis of MLLs was based on computed tomography (CT), and confirmed with biopsy in only two patients. In two other patients, the MLLs were diagnosed based on positron-emission-tomography-computed tomography (PET-CT). Ten inoperable patients with metastatic disease were considered as ineligible for either first-line or continuation of chemotherapy. Two patients with renal cell cancer (RCC), who had previously undergone pulmonary metastasectomy, refused further surgical resection for recurrent metastases in a different lung lobe. The majority of patients had one or two lung lesions (n=8; n=6, respectively), only three patients presented with three metastases. Two patients later received a second SABR for metachronous recurrent lesions. The primary tumors in the patients with MLLs were head and neck cancer (n=2), colorectal cancer (n=7), RCC (n=2), urothelial cancer (n=1), breast cancer (n=2), ovarian cancer (n=1), endometrial cancer (n=1) and sarcoma (n=1). At the time of SABR delivery, all these primary tumors were locally controlled, and all patients except for two patients with additional liver and brain metastases, which were simultaneously treated with liver and brain SABR—had no further extrathoracic manifestations.
PLLs were defined as a malignant lesion of pulmonary origin, i.e., either a first diagnosis of histologically proven or suspected non-small-cell lung cancer or pulmonary metastases in the context of a prior diagnosis of non-small-cell lung cancer. All cases of PLLs were either medically or technically inoperable. Twelve of 40 patients with PLL had a previous history of a primary lung cancer and were considered as recurrent (either locally or at a distant intrathoracic site), in five of whom the cancer was histologically proven, whereas in seven the diagnosis was based on CT (n=2) or PET-CT criteria (n=5). In this subgroup three patients recurred as multiple recurrent lung cancer (3 lesions, n=1; 2 lesions, n=2). Twenty eight patients with PLLs were de novo, in 24 of whom the cancer was histologically proven, whereas in 4 the diagnosis was based on PET-CT criteria. In five of these de novo cases, the disease manifested initially as multiple primary lung cancer (each had two lesions). FDG-PET staging was available in 36 patients with PLLs. In two patients with histologically proven PLLs, there was no pathologic metabolism on PET-CT.
Treatment technique
The treatment technique was previously described elsewhere (9). Each patient was immobilized in supine position in a dual vacuum BodyFIX system (Medical Intelligence, Schwabmuenchen, Germany). Planning CT scan of the chest was acquired with a 16-slice 4D-spiral-CT (Brilliance CT Big Bore, Philips, Best, The Netherlands). Gross tumor volume (GTV) was defined as the maximum intensity projection at each voxel during the entire respiratory cycle and was contoured in all ten phases. The GTVs were fused to create the internal target volume (ITV). The planning target volume (PTV) was generated by adding 5 mm to ITV in all directions. SABR treatment plans were created in the Philips Pinnacle3™ treatment planning system (TPS) v.08 and v.09 (Philips, Best, The Netherlands) using 6 MV photons. The dose distributions were calculated with a collapsed cone algorithm for heterogeneity corrections. Dose-volume-histograms were evaluated according to the dose-constraints suggested by the AAMP Task Group 101 (10). Patient alignment was verified by means of kV cone beam CT (CBCT) before each treatment. Translational set-up uncertainties up to 3 mm and rotations up to 3 degrees were tolerated.
Three-dimensional conformal treatment (3D) plans were used in 50 lesions, and intensity-modulated radiation therapy (IMRT) in 27 lesions. Routinely, three fractions of SABR were administered per week with a minimum interval between fractions of 40 hours. Different dose-fractionation schemes were applied with a median dose per fraction (prescribed to the isodose surrounding the PTV) of 12 Gy (range, 3.6–18 Gy) and a total dose of 48 Gy (range, 25–60 Gy) in 4 fractions (range, 3–10 fractions) delivered in 9 days (range, 5–23 days). The most common dose-fractionation schedules were 4×12 Gy (n=21), 5×12 Gy (n=15) and 8×7.5 Gy (n=12).
Endpoints and follow-up
Endpoints of this retrospective study were PFS, LC, SAFP, OS, timing and location of treatment failure, and treatment related late toxicity.
Six weeks after SABR, all patients underwent clinical examination and chest-CT. Then, they were followed every 3 months in the first 2 years, and every 6 months thereafter. PET-CT was performed only in case of differential diagnosis between recurrence and radiation-induced consolidation. Toxicity was assessed using Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
Time to any of the pre-defined events was calculated from the first day of SABR to the date of an event. Data were censored, when the patients showed no evidence of an event at their last follow-up during the period of analysis. Furthermore, survival after the first progression post-SABR (SAPF) was calculated from the date of the diagnosis of the first disease progression after SABR treatment to the date of the death or the loss of follow-up. For between-group comparison, the Pearson chi-square test was used for categorical and Mann-Whitney test for continuous variables. To estimate the rates of local failure, PFS and OS, the Kaplan-Meier method was used, and the differences between groups were compared by means of the log-rank test.
The uni- and multivariate Cox proportional hazards regression model was used to identify prognostic factors for endpoints. Tumor and treatment-related factors were used to assess the PFS and LC, whereas clinical factors were used to assess SAFP and OS. In the multivariate analysis, a stepwise selection of covariates was done and all predictors with P value <0.10 were retained in the final model. Continuous variables were dichotomized at the median value, or converted into categorical variables. Hazard ratio and the 95% confidence interval were also reported. All statistical tests were 2-sided, and P value <0.05 was considered significant. Data were analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
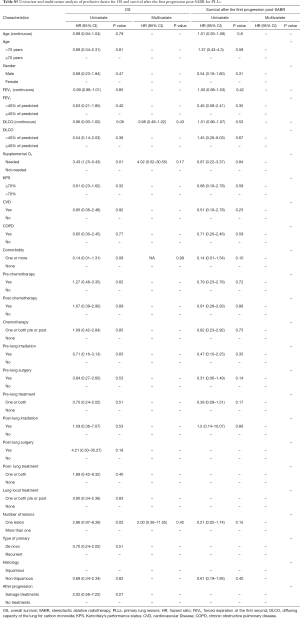
There was no difference between groups regarding age, gender, pre- and post-SABR lung local treatment (surgery, irradiation), post-SABR chemotherapy and cardiovascular disease (CVD). However, patients in the MLLs group had a significantly higher Karnofsky performance status (KPS) (P=0.01). Pretreatment lung function variables including forced expiratory volume during the first second (FEV1), diffusion capacity of the lung for carbon monoxide (DLCO) and the need of supplemental oxygen were also significantly better in patients with MLLs. There was a higher percentage of chronic obstructive pulmonary disease (COPD) in the PLLs group (75% vs. 12%, P=0.001). This difference remained significant, when COPD + CVD were considered as one variable (P=0.001). All patient basic characteristics are illustrated in Table 1.
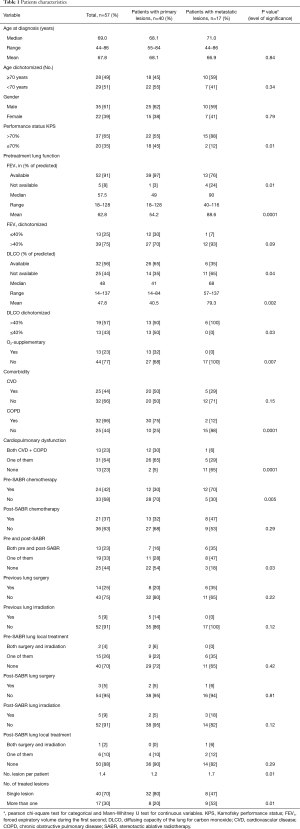
Full table
Lesion characteristics
There was a significant difference between MLLs and PLLs regarding the method by which the diagnosis was established (biopsy vs. PET vs. CT, P=0.0001), the histological type of cancer (squamous cell vs. adenocarcinoma vs. others, P=0.004), the anatomic location (P=0.003) and the topography of tumor in relation to organs at risks (OARS) (peripheral vs. parenchymal vs. central, P=0.002). Furthermore, GTV as a continuous or categorical variable was not significantly different between groups. The lesions’ characteristics are shown in Table 2.
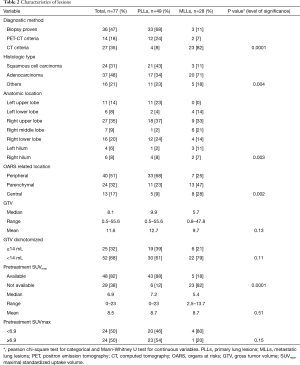
Full table
Treatment characteristics
Time interval, calculated from the date of first diagnosis of MLLs or PLLs to the first day of SABR, treatment duration, number of fractions and treatment planning techniques were not significantly different between groups. Although PTV volume was not significantly different between MLLs and PLLs, MLLs were—on average—treated with more conservative SABR-regimens with respect to total dose (P=0.001) and BED10 <105 (P=0.0001). The dose-fractionation in MLL-patients, in whom the toxicity and benefit were at that time unclear, has been more conservative. Further treatment characteristics are highlighted in Table 3.
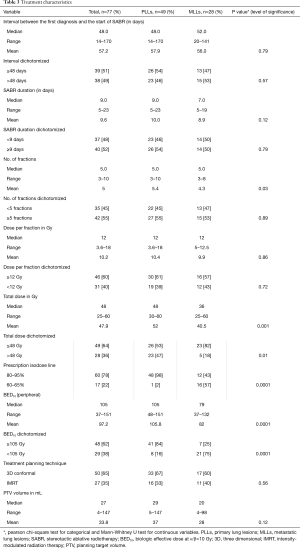
Full table
Temporal and locational distribution of the first disease progression
With a median follow-up of 14 months (range, 4–40 months), progression occurred in 22 of 49 (45%) PLLs compared with 18 of 28 (64%) MLLs (P=0.1). However, there was a significant difference in the time to progression between groups (P=0.003) with a median onset of 8 months (range, 6–24 months) in PLLs compared with 4.5 months (range, 1–26 months) for MLLs. There was no significant difference between MLLs from colorectal cancer and non-colorectal cancer (P=0.2) and between PLLs of squamous vs. non-squamous histology (P=0.29). The difference in progression was independent of the type of PLLs (de novo vs. recurrent, P=0.06).
The locational distribution of progression was also significantly different between groups (P=0.01). Whereas all types of progression were seen in PLLs, the pattern of failure in MLLs was mainly distant, either isolated or combined with local recurrence (LR). Post-SABR, the lung parenchyma was the predominant site for distant failure in both groups. In only two patients with MLLs and a prior history of extrapulmonary manifestations, additional metastases occurred in the brain from breast cancer and in the liver from colorectal cancer. In the PLLs groups, isolated extrapulmonary distant failure occurred in four patients (liver, n=1; bone, n=2; adrenal gland, n=1).
The main salvage treatment after first progression post-SABR in the MLL-group was chemotherapy (n=7) and a second course of SABR (n=2). In the PLLs patients, salvage chemotherapy was administered in seven patients and nine patients received no further treatment or best supportive care, respectively. The difference in receiving salvage treatments after the first progression was also significant (P=0.005). There was a significant difference regarding the percentage of deceased patients after the first progression post-SABR (75% for PLL vs. 34% for MLL, P=0.041).
LR was diagnosed in 11 PLLs and 6 MLLs (P=0.9). With a median onset of 9 months (range, 6–24 months) for PLLs and 3 months (range, 3–26 months) for MLLs, there was no significant difference in the time until LR (P=0.22) The diagnosis was based on biopsy in one patient with PLLs, PET-CT in 3 PLLs vs. 2 MLLs, and CT in 7 PLLs and 4 MLLs.
LC and PFS
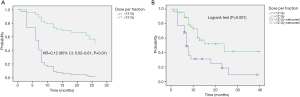
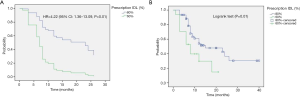
The actuarial 1- and 2-year LC rates were 79.3% (95% CI: 66.4–92%) and 52.6% (95% CI: 20.3–85%) in PLLs compared with 85.7% (95% CI: 72.8–98.7%) and 64.3% (95% CI: 26.7–85.4%) in MLLs, P=0.25 and P=0.47 respectively. There was no significant difference in the LR free survival (log-rank, P=0.98) (Figure 1).
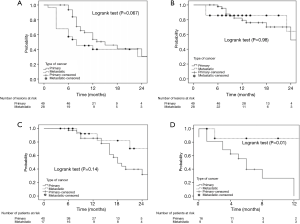
The actuarial 1- and 2-year PFS rates were 53.6% (95% CI: 37.5–69.6%) and 30.5% (95% CI: 9–52.4%) in PLLs compared with 40% (95% CI: 20.9–59.3%) and 30% (95% CI: 8–52.4%) in MLLs, P=0.37 and P=0.48. There was no significant difference in the PFS (log-rank, P=0.06) (Figure 1).
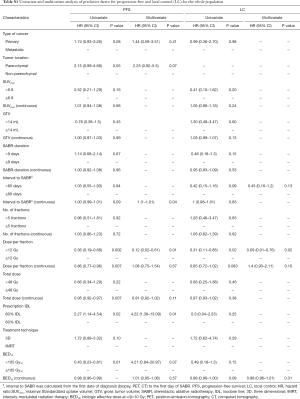
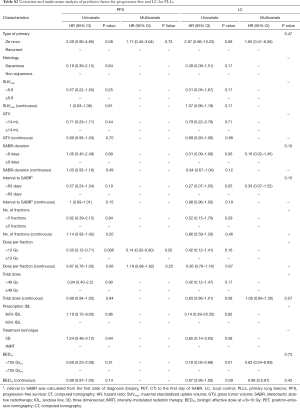
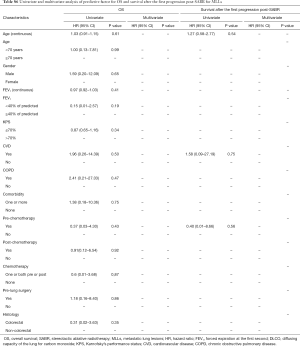
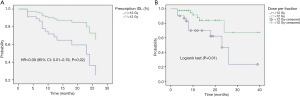
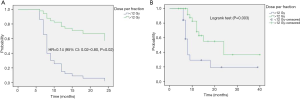
In the multivariate analysis, only dose per fraction (> or <12 Gy) was an independent prognostic factor for both LC (HR =0.09, 95% CI: 0.01–0.76, P=0.02) and PFS (HR =0.12, 95% CI: 0.02–0.61, P=0.01) for all patients, and remained predictive for PFS for the PLLs subgroup (HR =0.14, 95% CI: 0.02–0.8, P=0.02). No prognostic factors in the subgroup analysis for LR were found.
Survival after the first progression post-SABR and OS
MLLs patients had significantly better survival after progression post-SABR as compared to PLLs patients (log-rank, P=0.01) (Figure 1). The actuarial 6-month SAFP was 45% (95% CI: 17.3–73%) in PLLs patients and 85.7% in MLLs patients (95% CI: 59.8–100%), P=0.37.
There was no difference between groups regarding the median OS (log-rank, P=0.14) (Figure 1). The 1- and 2-year OS rates were 85.5% (95% CI: 73.8–97.3%) and 39.6% (95% CI: 20.2–59%) in PLLs compared with 92.3% (95% CI: 77.8–100%) and 70% (95% CI: 41–99%), P=0.5 and P=0.09 respectively.
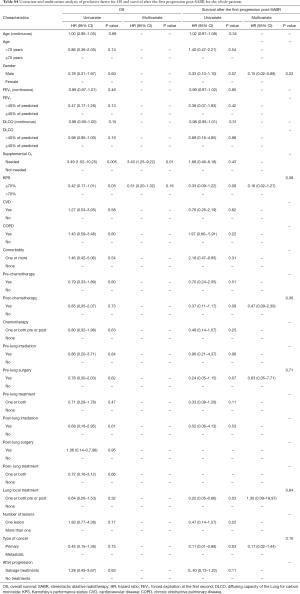
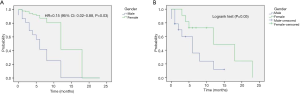
In the multivariate analysis, the gender was the only predictive factor for longer SAFP (HR =0.16; 95% CI: 0.02–0.89; P=0.036). For the whole population, the need for supplemental oxygen (HR =3.48; 95% CI: 1.26–9.56; P=0.016) was an independent predictive factor for worse OS.
To get more insight and entire view, univariate and multiple explorative statistical analyses including more than 45 covariates have been performed in addition. The interested reader is referred to supplementary appendix online (Tables S1-S7,Figures S1-S7).
Full table
Full table
Full table
Full table
Full table
Full table
Full table


Late toxicity
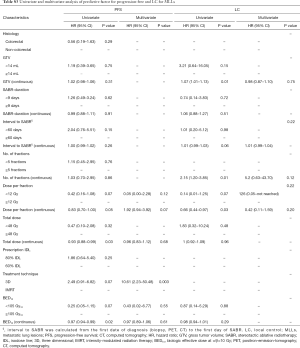
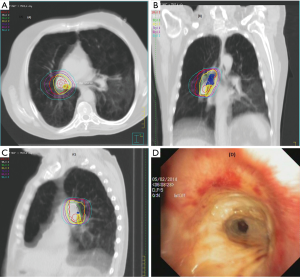
Adverse events after 90 days post-SABR occurred in six patients (10%) with PLLs. No long-term toxicity (grade ≥2) was observed in the MLLs group. There was a correlation between clinical symptoms and macroscopic damages caused in radiation in three of them (rib fracture, bronchial necrosis and radiation pneumonitis), whereas the other three toxicities were rather related to COPD exacerbation (dyspnea) (Table 4,Figure 2). Due to the low incidence of radiation pneumonitis, no DVH analysis was performed.
Full table
Discussion
The net benefit from SABR for MLLs remains unclear and has been basically extrapolated from the experience with early stage lung cancer. Patients with MLLs were frequently included in series on SABR for primary lung cancer, making the interpretation of treatment impact on the outcomes of SABR for this population difficult. Conversely, PLLs were also included in studies for MLLs. In a recent published systematic review (11), all five studies on stereotactic radiosurgery included lesions of pulmonary origins with a median percentage of 34% (range, 6–51%), and 11/13 studies of SABR included such lesions with a median percentage of 22% (range, 8–62%).
There have been a few studies that addressed the issue of comparison between MLL and PLL patients. Wulf et al. (5) compared the outcomes of 51 MLLs with that of 20 PLLs and found no difference in LC, freedom from systematic progression and OS. In another study from the same institution (6), where 118 MLLs were compared with 41 PLL, there was no difference in LC and OS at 3 years. Takeda et al. (7) compared the outcomes of 44 MLLs from colorectal cancer and other primary cancers with 115 PLLs, and found worse LC rate for MLLs compared with PLLs (P=0.001), and in the subgroup analysis worse LC for MLLs form colorectal cancer compared with MLLs of other origins. Similar results were found in another study (8), in which the LC between MLLs and PLLs was significantly different (P=0.01), and the difference in LC for MLLs from colorectal cancer was significant (P=0.022). In the presented study, LR occurred in lung metastases from ovary (n=2), sarcoma (n=2), and head and neck cancer (n=2).
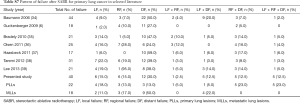
The locational distribution of the treatment failure are compared with published literature and illustrated in Table S7. In our study, the lung parenchyma was the common site for the first new metastases post-SABR. Milano and colleagues (12) reported in detail the patterns of recurrence after curative-intent radiation of oligometastases confined to one organ and found that the first new metastases in patients with MLLs occurred in the lung parenchyma (40% compared with 27% in a distant organ) with a median onset of 6 months (range, 3–69 months).
In concordance with the policy of other institutes (13,14) and because of the unknown toxicity and efficacy of SABR for oligometastases at the time of SABR implementation in our institute, more conservative dose-fractionation schedules were used for this cohort. Now in the absence of severe toxicity, and similar efficacy of SABR, we are moving toward treating MLLs and PLLs with the same dose-fractionation schedule.
Onishi et al. (15) from Japan were the first who described the SABR-thoracic dogma of BED10 >100 Gy as prerequisite for better LC for primary lung cancer. This concept was confirmed by another study (6), and extrapolated for MLLs (8,16). However, this dogma is based on chi-square test (15) and log rank (6) univariate analysis and remains therefore controversial (17). In a meta-analysis (18), the OS for medium BED10 (range, 83.2–105 Gy) was even as high as for medium to high BED10 (range, 106–146 Gy). Notwithstanding, our data showed similar dose-relationship on the univariate analysis. BED10 >105 Gy was found to be a significant predictor for PFS for the whole population (HR =0.43; 95% CI: 0.23–0.81; P=0.01) and for LC in the patients with PLLs (HR =0.18; 95% CI: 0.05–0.68; P=0.01). Furthermore, the PFS in the patients with MLLs was significantly better with increased BED10 as continuous variable (HR =0.97; 95% CI: 0.94–0.99; P=0.02). Multivariate analysis revealed the dose per fraction (≥ or <12 Gy) was an independent predictor for LC (P=0.02) and PFS (P=0.01) for the whole population, and for PFS (P=0.02) in the PLLs group. Indeed, there is evidence that LC may be improved with higher fractional dose. In a dose-escalation series on patients with lung and liver metastases (19), the LC was improved on the uni- and multivariate analysis with greater nominal dose, i.e., with greater dose per fraction, since the number of fractions remained constant. A similar observation was reported in patients with hepatocellular carcinoma (20). Timmerman et al. (21) observed in a dose-escalation study on early-staged primary lung cancer no treatment failures in patients treated with a dose greater than 18 Gy/fraction. Similarly, PLLs in the Stage IB were locally better controlled, when the dose was escalated from 10 to 12 Gy/fraction (22). In another study (23) on pulmonary and hepatic metastases from RCC and malign melanoma, Log rank comparison revealed dose per fraction (>11 vs. <11 Gy/fraction, P<0.01) to be significant predictors of LC. Similar results were reported in another study on metastatic RCC (24), in which a univariate analysis revealed dose per fraction ≥9 Gy (HR =0.631; 95% CI, 0.429–0.931; P=0.021) to be predictive factor for radiographic LC. The dose per fraction ≥9 Gy (HR =0.396; 95% CI, 0.163–0.962; P=0.042) was a significant predictor for clinical LC in another report as well (25). These data support the concept that a dose per fraction >10 Gy may independently of the histological type of the cancer induce severe vascular damage leading to indirect cell death (26).
The toxicity results are consistent with the published literature even for central MLLs (27). The most serious SABR complication in our study was a bronchial necrosis. Indeed, radiation necrosis has been infrequently reported in SABR-literature. There are other three cases of bronchial necrosis that resulted in different clinical scenarios, i.e., fatal hemoptysis (28), atelectasis (29) and bronchial fistula formation (30). Regarding the difficulty to distinguish SABR complications from those of exacerbation of cardiopulmonary comorbidity, the reader is referred to our previous discourse on this issue (31).
Our study had several limitations including the inherent risk of bias due to its retrospective nature, small sample size of MLLs, widely different treatment protocol, method of dose prescription, and variety of dose-fractionation schemes. One relevant bias is the questionable definition of LR based solely on CT-criteria in the majority of patients, which may have overestimated the rate of tumor LR (32,33).
Conclusions
Despite significant differences in patient, lesion and treatment-related characteristics, SABR for MLLs provided similar results without long-term toxicity, when compared with those of primary lung cancer. Dose per fraction ≥12 Gy as complementary to the concept of BED10 >100 Gy may be radiobiologically meaningful to explain the ablative potential of SABR.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: In response to our formal request and notification of the study, the responsible ethics committee (Ärztekammer des Saarlandes) waived the need for approval and patients’ informed consent for this retrospective study as the analysis was based on non-identifiable, anonymized data only. Written informed consent was obtained from all patients.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J Clin Oncol 2013;31:1384-90. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Punglia RS, Morrow M, Winer EP, et al. Local therapy and survival in breast cancer. N Engl J Med 2007;356:2399-405. [Crossref] [PubMed]
- Wulf J, Haedinger U, Oppitz U, et al. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004;60:186-96. [Crossref] [PubMed]
- Guckenberger M, Wulf J, Mueller G, et al. Dose-response relationship for image-guided stereotactic body radiotherapy of pulmonary tumors: relevance of 4D dose calculation. Int J Radiat Oncol Biol Phys 2009;74:47-54. [Crossref] [PubMed]
- Takeda A, Kunieda E, Ohashi T, et al. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011;101:255-9. [Crossref] [PubMed]
- Yamamoto T, Jingu K, Shirata Y, et al. Outcomes after stereotactic body radiotherapy for lung tumors, with emphasis on comparison of primary lung cancer and metastatic lung tumors. BMC Cancer 2014;14:464. [Crossref] [PubMed]
- Dzierma Y, Nuesken FG, Fleckenstein J, et al. Visualisation of respiratory tumour motion and co-moving isodose lines in the context of respiratory gating, IMRT and flattening-filter-free beams. PLoS One 2013;8:e53799. [Crossref] [PubMed]
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078-101. [Crossref] [PubMed]
- Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: a systematic review. J Thorac Oncol 2010;5:1091-9. [Crossref] [PubMed]
- Milano MT, Katz AW, Okunieff P. Patterns of recurrence after curative-intent radiation for oligometastases confined to one organ. Am J Clin Oncol 2010;33:157-63. [PubMed]
- Singh D, Chen Y, Hare MZ, et al. Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung. J Thorac Dis 2014;6:369-74. [PubMed]
- Gillespie EF, Atwood TF, Sandhu AP. Lung stereotactic body radiotherapy (SBRT): a single institution's outcomes and methodology in the context of a literature review. Transl Cancer Res 2015;4:372-80.
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Park S, Urm S, Cho H. Analysis of biologically equivalent dose of stereotactic body radiotherapy for primary and metastatic lung tumors. Cancer Res Treat 2014;46:403-10. [Crossref] [PubMed]
- Duncker-Rohr V, Nestle U, Momm F, et al. Stereotactic ablative radiotherapy for small lung tumors with a moderate dose. Favorable results and low toxicity. Strahlenther Onkol 2013;189:33-40. [Crossref] [PubMed]
- Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011;81:e305-16. [Crossref] [PubMed]
- McCammon R, Schefter TE, Gaspar LE, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2009;73:112-8. [Crossref] [PubMed]
- Jang WI, Kim MS, Bae SH, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol 2013;8:250. [Crossref] [PubMed]
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003;124:1946-55. [Crossref] [PubMed]
- Onimaru R, Fujino M, Yamazaki K, et al. Steep dose-response relationship for stage I non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:374-81. [Crossref] [PubMed]
- Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol 2011;6:34. [Crossref] [PubMed]
- Altoos B, Amini A, Yacoub M, et al. Local Control Rates of Metastatic Renal Cell Carcinoma (RCC) to Thoracic, Abdominal, and Soft Tissue Lesions Using Stereotactic Body Radiotherapy (SBRT). Radiat Oncol 2015;10:218. [Crossref] [PubMed]
- Amini A, Altoos B, Bourlon MT, et al. Local control rates of metastatic renal cell carcinoma (RCC) to the bone using stereotactic body radiation therapy: Is RCC truly radioresistant? Pract Radiat Oncol 2015;5:e589-96. [Crossref] [PubMed]
- Park HJ, Griffin RJ, Hui S, et al. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012;177:311-27. [Crossref] [PubMed]
- Nuyttens JJ, van der Voort van Zyp NC, Verhoef C, et al. Stereotactic body radiation therapy for oligometastases to the lung: a phase 2 study. Int J Radiat Oncol Biol Phys 2015;91:337-43. [Crossref] [PubMed]
- Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 2012;366:2327-9. [Crossref] [PubMed]
- Rowe BP, Boffa DJ, Wilson LD, et al. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol 2012;7:1394-9. [Crossref] [PubMed]
- Unger K, Ju A, Oermann E, et al. CyberKnife for hilar lung tumors: report of clinical response and toxicity. J Hematol Oncol 2010;3:39. [Crossref] [PubMed]
- Oskan F, Becker G, Bleif M. Specific toxicity after stereotactic body radiation therapy to the central chest: A comprehensive review. Strahlenther Onkol 2017;193:173-84. [Crossref] [PubMed]
- Takeda A, Kunieda E, Takeda T, et al. Possible misinterpretation of demarcated solid patterns of radiation fibrosis on CT scans as tumor recurrence in patients receiving hypofractionated stereotactic radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2008;70:1057-65. [Crossref] [PubMed]
- Dunlap NE, Yang W, McIntosh A, et al. Computed tomography-based anatomic assessment overestimates local tumor recurrence in patients with mass-like consolidation after stereotactic body radiotherapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;84:1071-7. [Crossref] [PubMed]
- Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol 2006;45:787-95. [Crossref] [PubMed]
- Bradley JD, El Naqa I, Drzymala RE, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys 2010;77:1146-50. [Crossref] [PubMed]
- Olsen JR, Robinson CG, El Naqa I, et al. Dose-response for stereotactic body radiotherapy in early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e299-303. [Crossref] [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [Crossref] [PubMed]
- Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012;82:967-73. [Crossref] [PubMed]
- Lee DS, Kim YS, Yoo IeR, et al. Long-term clinical experience of high-dose ablative lung radiotherapy: high pre-treatment [18F]fluorodeoxyglucose-positron emission tomography maximal standardized uptake value of the primary tumor adversely affects treatment outcome. Lung Cancer 2013;80:172-8. [Crossref] [PubMed]


