A single center experience: rituximab plus cladribine is an effective and safe first-line therapy for unresectable bronchial-associated lymphoid tissue lymphoma
Introduction
Extranodal marginal zone lymphoma of the lung, also called bronchus-associated lymphoid tissue (BALT) lymphoma, is an indolent B-cell non-Hodgkin lymphoma (B-NHL) that originates from mucosa-associated lymphoid tissue (MALT) of bronchus. Pulmonary lymphoma is a rare entity accounts for only 0.4% of all non-Hodgkin lymphomas (NHL) (1), and BALT lymphoma is the most common histologic subtype, representing 70–90% of pulmonary NHL (2,3). BALT is characterized by organized lymphoid aggregates located in pulmonary parenchyma, and its development is considered to be triggered by various chronic antigen stimulation related to autoimmune process, persistent infection or toxic exposure (4). Additional genetic alterations, for instance, t(11;18)(q21;q21), contribute to the transformation of this reactive extranodal lymphoid tissue to lymphoma (5).
In most cases, patients with BALT lymphoma are asymptomatic and pulmonary lesions are discovered via a routine chest radiographic study incidentally. In symptomatic patients, non-specific pulmonary symptoms (such as cough, sputum, chest pain and dyspnea) are the most common (6). Radiographic findings are usually non-specific, including nodules, air space consolidation with or without bronchogram, patchy opacities and pleural effusion (6,7). The clinical role of fluorine-18-fluorodeoxyglucose positron emission tomography (18F-FDG PET-CT) in BALT lymphoma remains unclear, but several studies have reported high detection rate in BALT lymphoma suggesting PET-CT might be a promising tool in initial evaluation and response assessment of BALT lymphoma (8-10). Accurate diagnosis of BALT lymphoma depends upon histopathologic studies including morphological observation and immunohistochemical staining on biopsy specimen obtained by bronchoscopies, percutaneous lung biopsies or surgical resection (11). Benefiting from current findings revealing the pathogenesis of MALT lymphoma, cytogenetic studies, such as polymerase chain reaction (PCR) detecting clonal immunoglobulin heavy chain (IgH) gene rearrangement and fluorescence in situ hybridization (FISH) for MALT1 gene translocation, have been more and more widely applied in the diagnostic process of BALT lymphoma (12,13).
Currently, there is no uniform treatment for BALT lymphoma, partially due to the limitation of small population and heterogeneity of reported series. Thoracic surgery may play critical role in both diagnostic process and therapeutic approach; however, with the improvement of diagnostic modalities, such as CT-guided percutaneous lung biopsies and cytogenetic studies, the diagnostic value of surgery has been weakened. Although lung surgery has been reported to result in long-term disease-free survival for patients with localized disease (6,14), surgical resection is not recommended as first-line therapy unless the lesion is localized and a wedge resection or middle lobe and lingula excision are possible (15), mainly because the clinical risks like thoracic pain and lung function impairment observed in 10–15% patients underwent surgery (3). For patients with bilateral lesions and multi-lobar involvement, surgical resection is not indicated and systemic therapy is the only proper way to treat the disease. Various chemotherapeutic/immune-chemotherapeutic agents and combination regimens, including chlorambucil, mitoxantrone, CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone), CHOP-like, or fludarabine-containing regimens, with or without rituximab, have demonstrated some activity in the treatment of BALT lymphoma, but standard regimen is still debated (7,15-20). In a previously reported retrospective study on 205 patients with BALT lymphoma (6), most patients in relapse after incomplete surgical excision or patients with advanced disease received systemic treatment of alkylating-containing regimens with or without rituximab. However, the overall responses were not so satisfying, with an overall response rate (ORR) ranging from 80% to 87% (chemotherapy alone versus immunochemotherapy with rituximab). Hence, there exist urgent needs to find out a better regimen for those patients. Both the anti-CD20 monoclonal antibody rituximab (R) and the nucleoside analogue 2-chlorodeoxyadenosine (cladribine, 2-CdA) have demonstrated high efficacy and minimal side effects in the treatment of MALT lymphoma (21-23). The combination of rituximab and cladribine (R-2-CdA) has also been tested and showed promising results in both non-gastric MALT lymphoma and refractory/relapsed indolent B-NHL (24-26). Nonetheless, the effect and safety of R-2-CdA therapy in untreated BALT lymphoma with unresectable advanced lesions had not been assessed. In view of this, we performed this retrospective study to critically review homogenous data of patients with stage IV BALT lymphoma treated in first-line therapy with R-2-CdA regimen. It is also the first research focusing on the systemic therapy with rituximab and cladribine for previously untreated patients with unresectable advanced stage BALT lymphoma.
Methods
Patients
This is a retrospective analysis of 8 patients diagnosed with BALT lymphoma who were treated in first-line therapy with cladribine and rituximab chemoimmunotherapy at Zhongshan Hospital of Fudan University. Medical records of patients followed between November 2014 and September 2016 were reviewed.
In all cases, histological examination and adequate immunophenotyping analysis were performed on tissue samples obtained from bronchoscopic biopsies, CT-guided or ultrasound-guided percutaneous lung biopsies, and diagnosis was made according to the criteria highlighted in the World Health Organization (WHO) classification for tumors of hematologic and lymphoid tissues (27). Additional cytogenetic studies including both PCR detecting clonal IgH gene rearrangement and FISH identifying MALT1 gene translocation were performed to provide supplementary diagnostic information.
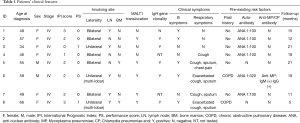
The following clinico-pathological data were collected: age, gender, clinical symptoms, previous history of pulmonary disease, performance status, clinical stage, extend of extranodal involvement, nodal involvement, bone marrow (BM) involvement, International Prognostic Index (IPI), immunofixation electrophoresis, autoimmune status (including autoantibodies and history of autoimmune disorders), microbial infection status for Helicobacter pylori, Chlamydia pneumoniae (CP) and Mycoplasma pneumonia (MP). Pulmonary function test (PFT) was performed on selected patients who have obvious respiratory symptom in the onset of disease. The IPI score was recorded according to the published criteria (28).The staging was based upon the Ann Arbor staging system (29).
Every patient underwent adequate clinical evaluation at diagnosis, in the interim of treatment, at the end of therapy and during post-treatment follow-up, with an assessment procedure at the minimum comprising a physical examination, hematological and chemical laboratory surveys, bronchoscopy, and positron emission tomography-computed tomography (PET-CT) [or contrast-enhanced computed tomography (CT) of chest, abdomen and pelvis]. Additional BM biopsy was performed on patients with BM involvement to assess response to therapy. Response was evaluated after 2 cycles of therapy and after completion of all scheduled therapy; according to the 2007 International Working Group revised response criteria (30). Patients were classified according to best tumor response: complete response (CR), partial response (PR), stable disease (SD), or disease progression (PD). The ORR was calculated as the proportion of patients who obtained a CR or PR. Toxicities and side-effects were documented and were graded regarding to the Common Toxicity Criteria for Adverse Events v3.0 (31).
The study was conducted in accordance with the Declaration of Helsinki. All patients were de-identified and their medical data was analyzed anonymously. Oral informed consent was obtained from all participants via telephone or face-to-face interview for the use of their anonymized medical records for research purpose. Zhongshan Hospital ethics committee approved the analysis and all medical record review.
Statistical analysis
All the statistical analyses were conducted with SPSS Statistics version 21.0. Patient characteristics are presented as descriptive statistics, with counts and percentages for categorical variables and median (range) for continuous variables. Kaplan-Meier survival analysis was performed to obtain estimate of overall survival (OS) and progression-free survival (PFS). OS was measured from time of diagnosis to date of last follow-up or death from any cause. PFS was calculated from time of diagnosis to the time of disease progression/relapse or death as a result of any cause. Duration of follow-up was measured from the date of diagnosis to the last follow-up or date of death.
Results
Patient characteristics
We performed a computer-based search in the electronic registry of Zhongshan hospital. A total of 8 patients with biopsy-proven BALT lymphoma treated with R-2-CdA regiment as first-line therapy in the period from January 2007 to September 2016 were identified. Patients were enrolled consecutively in purpose of avoiding selection bias.
Table 1 summarizes the patient characteristics at diagnosis. The median age at diagnosis was 57 years, ranging from 22 to 82 years. Six were female and the remaining 2 were male. At study entry, every patient had a good performance status (ECOG PS 0-1). Low-titer antinuclear antibodies (1:100 to 1:320) were detected in 6 out of 8 patients (75%), whereas none of them had a history of autoimmune disease. In the onset of disease, 5 patients (62.5%) presented non-specific respiratory symptoms, including cough, sputum and chest pain, among whom two had a history of chronic obstructive pulmonary disease (COPD) and one was positive for anti-MP antibodies. Five patients presented hypoxemia, among which 3 of them had low to moderate ventilation dysfunction in PFT, one with slight restrictive ventilation disorder, and 1 with normal PFT result. The remaining 3 cases are asymptomatic at diagnosis. MALT1 gene rearrangement was detected in 5 out of 8 patients, and clonal IgH gene rearrangement was confirmed by PCR in 5 out of 6 tested patients.
Full table
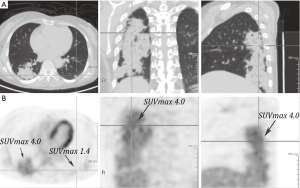
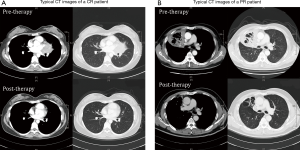
All the 8 patients were staged as stage IV according to Ann Arbor system. Regarding to the location of pulmonary lesion, 5 located bilaterally, and 3 located unilaterally with multi-lobar presentation. In all but two patients, the disease disseminated to extra-pulmonary tissue, 5 patients had lymph node (LN) involvement and 2 had BM involvement. Seven patients underwent PET-CT scanning at initial evaluation and all revealed low or moderate FDG uptake elevation [maximum standardized uptake values (mSUV) ranging from 4.0 to 8.8] in tumor lesions. (Typical images showed in Figure 1).
Treatment
All the 8 patients received well-scheduled R-2-CdA therapy. In accordance with our institutional guideline, the indications of immuno-chemotherapy were as followed: the treatment of patients with symptoms caused by lymphoma; asymptomatic patients with unresectable bilateral/multi-lobar pulmonary lesions, disseminated LN involvement or BM involvement; patients presented rapid progression requiring an intervention.
The R-2-CdA therapy consisted of rituximab (MabThera®, Roche China) administrated at a dose of 375 mg/m2 intravenously on day 1 of every cycle and cladribine (Aiboding®, HiSun Pfizer Pharmaceuticals CO., China) given at a dose of 0.1 mg/kg by IV injection on days 1−4. Before the administration of rituximab, a premedication consisted of 100 mg methylprednisolone was given intravenously. All patients received an intravenous pretreatment of 5-HT3 antagonist (either tropisetron or ondansetron IV) right before the cladribine. The R-2-CdA regimen was administered in 21-day cycles up to a maximum of six cycles. All treatment was given in day-care unit. All but one patient completed six cycles, whereas for the remaining patient, four cycles of R-2-CdA regimen followed by two cycle of rituximab monotherapy (375 mg/m2 IV every 21 days) were given due to a prolonged leukopenia. Prophylaxis with antiviral drugs (acyclovir 400 mg twice daily) was given to all the patients. Granulocyte-colony-stimulating factor (G-CSF) support was not performed routinely.
Routine evaluation, which comprised by a physical examination, complete blood counts, renal and hepatic parameters, was monitored before each cycle. Restaging was performed after every two cycles of therapy. Follow-up assessment consisted of PET-CT or contrast-enhanced CT of chest, abdomen and pelvis was performed every three months in the first year after the end of treatment and every six months in the second year. Additional bronchoscopy during follow-up was given to the patient whom was initially diagnosed by bronchoscopic biopsy.
Response and follow-up
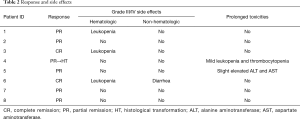
The response information for each patient was detailed in Table 2. All patients responded to RC regimen. Two patients achieved CR; the remaining had PR, resulting in an ORR of 100%. In particular, among the two patients who obtained CR, one showed complete resorption of tumor lesions with negative PET-CT scanning result immediately after four cycles of R-2-CdA therapy, whereas the other one presented slow respond to the treatment and achieved CR one year after the final administration (Figure 2). It is noteworthy that, among the five patients who impaired respiratory function at diagnosis, three were given additional PFT during interim or end-of-treatment evaluation, and all tested patients showed an ameliorated PFT result, even in patients with history of COPD (Table 3). All of the three patients recovered from hypoxemia, with significant improvement of total lung capacity (TLC) and diffusion capacity of carbon monoxide (DLCO) in two, and improved forced expiratory volume in 1 second (FEV1) in one.
Full table

Full table
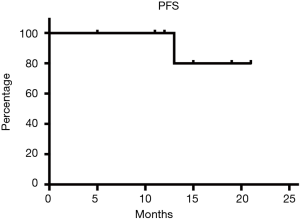
After a median follow-up of 16.5 months (interquartile range, 5–21 months), one patient endured disease progression 10 months after achieving PR. A bronchoscopy biopsy confirmed a histological transformation (HT) to large B cell lymphoma in this patient. To be noted, the PD patient had a negative PET-CT scan result during end-of-treatment evaluation, whereas simultaneous bronchoscopy detected residual lesion. Currently, all the patients are alive, and 7 of 8 (87.5%) subjects were in continuous CR/PR. The estimated PFS was 80% (Figure 3).
Toxicity
In total, 46 cycles of R-2-CdA therapy were given to the 8 patients; all patients but one (patient 8 did only four courses followed by two courses of rituximab monotherapy) completed six cycles. Treatment-related toxicities were generally mild and tolerable (Table 2). Hematologic toxicities were the main adverse event we observed, with grade III and IV leukopenia in 3/8 (37.5%) patients. After receiving adequate administration of G-CSF, all these patients recovered well from severe leukopenia. However, one patient, the one who had large cell transformation 10 months after PR, did develop prolonged grade III thrombocytopenia and grade I to II leukopenia. Further BM aspiration and biopsy did not show evidence of secondary myelodysplastic syndrome in this patient.
Grade III diarrhea was documented in one patient. One patient developed a grade II allergic reaction demonstrated by papular urticarial during infusion of rituximab. Grade I to II fever was observed in two patients without other obvious symptoms or underlying leukopenia. Two patients had slight hepatic dysfunction, one recovered fully after treatment and one had endured grade I aminotransferase elevation at the latest follow-up.
No episodes of febrile neutropenia resulting in extended hospitalization occurred, nor did cases of hepatitis B virus reactivation develop.
Discussion
Currently, the optimal treatment for pulmonary MALT lymphoma is still under debate and no global consensus has been reached. As opposed to gastric MALT lymphoma, the incidence of BALT lymphoma is comparatively low, and thus restrains the feasibility of large-scale prospective randomized controlled trial on this disease and limits the level of evidence for published related studies. In a retrospective analysis performed by the Korean Consortium for Improving Survival of Lymphoma involving a large international cohort of 205 patients with BALT lymphoma (6), localized approach, mainly surgical resection, resulted in improved PFS for patients with resectable lesion or early-stage disease, as compared to systemic treatment, and therefore led to a conclusion that systemic treatment can be reserved for patients with unresectable advanced disease and patients in relapse. However, according to the practice Guidelines for the management of extranodal NHL proposed by the Italian Society of Hematology (15), first-line treatment for pulmonary MALT lymphoma should include chlorambucil, CHOP, CHOP-like or fludarabine-containing regimens, as a recommendation with grade B evidence; in contrast, local approach, including surgery and radiation, is not recommended as first-line therapy (grade D). Furthermore, in a retrospective study of a cohort of 61 patients (20), the authors confirmed no difference in time to progression (TTP) between surgery group and chemotherapy; hence, they suggested chemotherapy should be the first-line therapy for BALT lymphoma in the purpose of avoiding the risk of operation and conserving lung function. Attributing to the novel understanding in the pathogenesis and genetics of the disease together with the development of precise diagnostic tools, the risk adapted therapeutic concepts, which encouraging organ preservation and focusing more on quality of life, had been proposed and widely accepted. Given these, systemic therapy with satisfying efficacy and mild toxicities seems to be a better choice for patients with advance-stage BALT lymphoma.
Both the monoclonal anti-CD20 antibody rituximab and the nucleoside analogue cladribine have demonstrated certain efficacy in the management of B-NHLs and some efficacy data was also reported in MALT lymphoma (22,23,26,32-34). Cladribine is a deoxyadenosine purine nucleoside analogue that has potent cytotoxic effects on both resting and proliferating lymphocytes (35,36). Monotherapy of cladribine is now considered the standard first-line treatment for hairy cell lymphoma (37,38). In addition, cladribine had shown satisfying effects in other indolent type of B-cell neoplasms, including marginal zone lymphoma, both as a single agent and in combination with other agents (22,24,25,34). Prolonged efficacy and long-term safety had been reported for cladribine in several publications (23,26).
Undoubtedly, the role of rituximab in the management of B-cell neoplasms is essential, especially in diffuse large B-cell lymphoma and follicular lymphoma. Although its use in MALT lymphoma has not been officially approved for this indication in China, rituximab has been reported to have high effectiveness in this disease, both in monotherapy and in combination immune-chemotherapy (21,33,39-42). Furthermore, according to a recent histologic study performed on a patient treated rituximab before receiving thoracic surgery for non-small cell lung cancer, rituximab showed its capability of penetration into lung tissue, and its high efficiency in depleting B cells in normal lung tissue, lung tumor and lung-related LNs (43). Given these, the potential therapeutic effect of rituximab in treatment of pulmonary MALT lymphoma appears to be promising.
Recently, there come several pivotal trials and retrospective studies assessing the efficacy and safety of the combination of rituximab and cladribine in MALT lymphoma. Hirokazu et al. reported high efficacy of R-2-CdA therapy as a salvage approach relapsed or refractory indolent B-NHL, with a ORR of 90% and CR of 70%. However, the 20-patient cohort in this study was mainly comprised by patients with follicular lymphoma, and only consisted 2 with MALT lymphoma (25). In a retrospective study involving 89 patients with marginal zone lymphoma, the use of R-2-CdA therapy resulted in an ORR of 89.3%, with 53.6% CR and 60 months of median of TTP, and a trend of early relapse in patients who did not receive rituximab was reported (44). Data of a total 16 patients with MALT lymphoma was analyzed in this study, whereas no BALT lymphoma was included. In 2013, Troch et al. published the first prospective non-randomized trial assessing the efficacy of the combination of rituximab and subcutaneous cladribine in MALT lymphoma (24). In this study of a 43-patient cohort, which contained 21 cases of gastric MALT lymphoma and 19 non-gastric cases (a total of 6 pulmonary MALT included), an CR of 58% and an ORR of 81% were reported; in particular, the response rate is significantly higher in patients with gastric MALT lymphoma versus non-gastric MALT (90% ORR with 16 CR and 2 PR versus 74% ORR with 7 CR and 7 PR), as also seen in a trial of cladribine alone (22). However, current published studies contained limited data regarding the use of R-2-CdA therapy in first-line treatment of BALT lymphoma, especially for advanced-stage disease.
To our best knowledge, our study is the first series that evaluated the efficacy and safety of R-2-CdA therapy as first-line therapy for chemotherapy-naïve patients with unresectable advanced-stage pulmonary MALT lymphoma. The study cohort is homogenous. All of the 8 patients in our cohort displayed disseminated distribution of tumor lesions at diagnosis and were consequently staged as stage IV in Ann Arbor system. Our data clearly illustrated that the combination of rituximab and cladribine in advanced BALT lymphoma is highly effective, resulting in an ORR of 100% with 2 CR and 6 PR. However, despite the high ORR, the complete response rate is slightly inferior to that reported in previous study by Troch et al., in which the CR was 36.8% (7/19) in the non-gastric subgroup and much lower than that of gastric subgroup (CR 80%, 16/20) (24), and lower than the CR (ranging from 45 to 100%) reported in a review of BALT lymphoma by Zinzani et al. (15). The difference in disease nature and pathogenic mechanism, for instance, autoimmune disorders in non-gastric MALT lymphoma versus HP infection in gastric subtype, might partially explain the inferior CR in BALT lymphoma when comparing with other non-gastric subtype and gastric subtype. In addition, the comparatively lower complete response rate can be explained by the advanced stage of disease in our cases. In our study, all the patients enrolled had stage IV diseases, among whom 5 had bilateral pulmonary lesions and 3 had unilateral involvement with multi-lobar presentation. Therefore, surgery and radiotherapy, which have been proved to be highly effective in localized pulmonary MALT lymphoma, are not indicated for the patients in our study due to their advanced stage, and systemic chemotherapy is the only choice. However, all the other studies (17,45,46) reporting a higher CR included patients who were treated by surgical resection or who had early stage disease. The difference in CR became smaller when the bias caused by surgical treatment and early stage were ruled out. Another possible explanation lies in the difference in response assessing modalities. While the feasibility of repeated endoscopic histological assessment insures accurate response evaluation in gastric lymphoma, radiographic examination remains to be the major response assessing tool for BALT lymphoma, which could lead to underestimating of CR due to irreversible structural lung injury or remnants of scar tissue in the lung.
Considering the limitation of traditional radiographic study in response evaluation, we further appraised the role of FDG PET-CT in initial staging and response evaluation. In fact, the results of current published studies related to PET-CT in MALT lymphoma were rather contradictory, some reported high sensitivity (8) and some denied the effect of detecting lymphoma lesion and reported high false positive rate (9). However, according to a recent meta-analysis, BALT lymphomas is (18) F-FDG-avid in most of the cases and PET-CT showed a high detection rate (98%) for this certain disease entity (10). In our study, all the 7 patients who underwent PET-CT at initial staging displayed increase FDG uptake, confirming the FDG-avidity of BALT lymphoma as reported before and suggesting the potential use in exact staging at diagnosis. During interim and end-of-treatment evaluation, every tested patient showed decrease in FDG uptake and disappearance of abnormal FDG uptake was observed in two patients. However, in one patient with negative PET-CT result, bronchoscopic biopsy demonstrated remnant of lymphoma tissue, suggesting that PET-CT is not sensitive for detecting small lesion and might be insufficient for response evaluation in BALT lymphoma in the absence of additional bronchoscopy or other radiographic study.
Noteworthily, we observed obvious improvement in pulmonary function after R-2-CdA therapy in patients who have impaired respiratory function at diagnosis. Hypoxemia was adjusted in all affected patients and FVC, DLCO, FEV1 increased after treatment in some patients. Despite the limitation of sample size, our findings support that chemotherapy might be superior than surgery for BALT lymphoma, in terms of preserving pulmonary function and avoiding operation-related risk.
After a median follow up of 16.5 months, one patient developed disease progression, presenting by large cell HT, 10 months after achieving PR. We are looking forward to long-term result after a longer follow-up period in the future.
We observed mild and well-tolerated toxicities in our study. Side effects were mainly manageable hematologic toxicities, with grade III and IV leukopenia in 3/8 (37.5%) patients. Secondary hematological malignancy, especially secondary myelodysplastic syndrome, is the major concern in the application of cladribine. In our series, two patients developed prolonged cytopenia. Although one patient recovered fully without any severe complications and the thrombocytopenia occurred in the second patient was asymptomatic and did not require platelet transfusion, we still suggested the necessity for well-monitoring of the myelotoxicity of cladribine in a prolonged observation time. As every patient received anti-viral prophylaxis, no event of herpes zoster reactivation was recorded in our study, which was not a rare event in the series of Troch et al. (24).
Considering the relatively indolent nature and favorable outcome of MALT lymphoma, how to find the balance between disease curation, symptoms controlling, minimizing treatment-related side effects and improving quality of life had been raised as a critical issue. In our series, the combination of rituximab and cladribine demonstrated to be effective and safe in newly diagnosed unresectable BALT lymphoma with advanced-stage disease. Although further large-scale study is needed for consolidation, R-2-CdA regimen could be considered as a proper first-line approach for patients with advanced-stage BALT lymphoma.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (81170486, 81570123).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by ethics committee board of Zhongshan Hospital (No. 2010-199) and written informed consent was obtained from all patients.
References
- Isaacson PG, Spencer J. Malignant lymphoma of mucosa-associated lymphoid tissue. Histopathology 1987;11:445-62. [Crossref] [PubMed]
- Chilosi M, Zinzani PL, Poletti V. Lymphoproliferative lung disorders. Semin Respir Crit Care Med 2005;26:490-501. [Crossref] [PubMed]
- Ferraro P, Trastek VF, Adlakha H, et al. Primary non-Hodgkin's lymphoma of the lung. Ann Thorac Surg 2000;69:993-7. [Crossref] [PubMed]
- Bienenstock J, McDermott MR. Bronchus- and nasal-associated lymphoid tissues. Immunol Rev 2005;206:22-31. [Crossref] [PubMed]
- Zhang Y, Wei Z, Li J, et al. Molecular pathogenesis of lymphomas of mucosa-associated lymphoid tissue--from (auto)antigen driven selection to the activation of NF-kappaB signaling. Sci China Life Sci 2015;58:1246-55. [Crossref] [PubMed]
- Sammassimo S, Pruneri G, Andreola G, et al. A retrospective international study on primary extranodal marginal zone lymphoma of the lung (BALT lymphoma) on behalf of International Extranodal Lymphoma Study Group (IELSG). Hematol Oncol 2016;34:177. [Crossref] [PubMed]
- Huang H, Lu ZW, Jiang CG, et al. Clinical and prognostic characteristics of pulmonary mucosa-associated lymphoid tissue lymphoma: a retrospective analysis of 23 cases in a Chinese population. Chin Med J (Engl) 2011;124:1026-30. [PubMed]
- Alinari L, Castellucci P, Elstrom R, et al. 18F-FDG PET in mucosa-associated lymphoid tissue (MALT) lymphoma. Leuk Lymphoma 2006;47:2096-101. [Crossref] [PubMed]
- Park SH, Lee JJ, Kim HO, et al. 18F-Fluorodeoxyglucose (FDG)-positron emission tomography/computed tomography in mucosa-associated lymphoid tissue lymphoma: variation in 18F-FDG avidity according to site involvement. Leuk Lymphoma 2015;56:3288-94. [Crossref] [PubMed]
- Treglia G, Zucca E, Sadeghi R, et al. Detection rate of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with marginal zone lymphoma of MALT type: a meta-analysis. Hematol Oncol 2015;33:113-24. [Crossref] [PubMed]
- Li G, Hansmann ML, Zwingers T, et al. Primary lymphomas of the lung: morphological, immunohistochemical and clinical features. Histopathology 1990;16:519-31. [Crossref] [PubMed]
- Kido T, Yatera K, Noguchi S, et al. Detection of MALT1 gene rearrangements in BAL fluid cells for the diagnosis of pulmonary mucosa-associated lymphoid tissue lymphoma. Chest 2012;141:176-82. [Crossref] [PubMed]
- Ko HM, Geddie WR, Boerner SL, et al. Cytomorphological and clinicopathological spectrum of pulmonary marginal zone lymphoma: the utility of immunophenotyping, PCR and FISH studies. Cytopathology 2014;25:250-8. [Crossref] [PubMed]
- Wang L, Xia ZJ, Zhang YJ, et al. Radical surgery may be not an optimal treatment approach for pulmonary MALT lymphoma. Tumour Biol 2015;36:6409-16. [Crossref] [PubMed]
- Zinzani PL, Martelli M, Poletti V, et al. Practice guidelines for the management of extranodal non-Hodgkin's lymphomas of adult non-immunodeficient patients. Part I: primary lung and mediastinal lymphomas. A project of the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica 2008;93:1364-71. [Crossref] [PubMed]
- Zinzani PL, Pellegrini C, Gandolfi L, et al. Extranodal marginal zone B-cell lymphoma of the lung: experience with fludarabine and mitoxantrone-containing regimens. Hematol Oncol 2013;31:183-8. [Crossref] [PubMed]
- Zinzani PL, Tani M, Gabriele A, et al. Extranodal marginal zone B-cell lymphoma of MALT-type of the lung: single-center experience with 12 patients. Leuk Lymphoma 2003;44:821-4. [Crossref] [PubMed]
- Zucca E, Conconi A, Pedrinis E, et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood 2003;101:2489-95. [Crossref] [PubMed]
- Kurtin PJ, Myers JL, Adlakha H, et al. Pathologic and clinical features of primary pulmonary extranodal marginal zone B-cell lymphoma of MALT type. Am J Surg Pathol 2001;25:997-1008. [Crossref] [PubMed]
- Oh SY, Kim WS, Kim JS, et al. Pulmonary marginal zone B-cell lymphoma of MALT type--what is a prognostic factor and which is the optimal treatment, operation, or chemotherapy?: Consortium for Improving Survival of Lymphoma (CISL) study. Ann Hematol 2010;89:563-8. [Crossref] [PubMed]
- Raderer M, Jager G, Brugger S, et al. Rituximab for treatment of advanced extranodal marginal zone B cell lymphoma of the mucosa-associated lymphoid tissue lymphoma. Oncology 2003;65:306-10. [Crossref] [PubMed]
- Jäger G, Neumeister P, Brezinschek R, et al. Treatment of extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type with cladribine: a phase II study. J Clin Oncol 2002;20:3872-7. [Crossref] [PubMed]
- Jäger G, Neumeister P, Quehenberger F, et al. Prolonged clinical remission in patients with extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type treated with cladribine: 6 year follow-up of a phase II trial. Ann Oncol 2006;17:1722-3. [Crossref] [PubMed]
- Troch M, Kiesewetter B, Willenbacher W, et al. Rituximab plus subcutaneous cladribine in patients with extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue: a phase II study by the Arbeitsgemeinschaft Medikamentose Tumortherapie. Haematologica 2013;98:264-8. [Crossref] [PubMed]
- Nagai H, Ogura M, Kusumoto S, et al. Cladribine combined with rituximab (R-2-CdA) therapy is an effective salvage therapy in relapsed or refractory indolent B-cell non-Hodgkin lymphoma. Eur J Haematol 2011;86:117-23. [Crossref] [PubMed]
- Kiesewetter B, Dolak W, Simonitsch-Klupp I, et al. Long-term safety and activity of cladribine in patients with extranodal B-cell marginal zone lymphoma of the mucosa-associated lymphoid tissue (MALT) lymphoma. Hematol Oncol 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol 2000;13:193-207. [Crossref] [PubMed]
- A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993;329:987-94. [Crossref] [PubMed]
- Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 1971;31:1860-1. [PubMed]
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Oh SY, Kim WS, Kim JS, et al. Stage IV marginal zone B-cell lymphoma--prognostic factors and the role of rituximab: Consortium for Improving Survival of Lymphoma (CISL) study. Cancer Sci 2010;101:2443-7. [Crossref] [PubMed]
- Zucca E, Conconi A, Laszlo D, et al. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study. J Clin Oncol 2013;31:565-72. [Crossref] [PubMed]
- Sigal DS, Miller HJ, Schram ED, et al. Beyond hairy cell: the activity of cladribine in other hematologic malignancies. Blood 2010;116:2884-96. [Crossref] [PubMed]
- Carson DA, Wasson DB, Taetle R, et al. Specific toxicity of 2-chlorodeoxyadenosine toward resting and proliferating human lymphocytes. Blood 1983;62:737-43. [PubMed]
- Leoni LM, Chao Q, Cottam HB, et al. Induction of an apoptotic program in cell-free extracts by 2-chloro-2'-deoxyadenosine 5'-triphosphate and cytochrome c. Proc Natl Acad Sci U S A 1998;95:9567-71. [Crossref] [PubMed]
- Hoffman MA, Janson D, Rose E, et al. Treatment of hairy-cell leukemia with cladribine: response, toxicity, and long-term follow-up. J Clin Oncol 1997;15:1138-42. [Crossref] [PubMed]
- Rosenberg JD, Burian C, Waalen J, et al. Clinical characteristics and long-term outcome of young hairy cell leukemia patients treated with cladribine: a single-institution series. Blood 2014;123:177-83. [Crossref] [PubMed]
- Kiesewetter B, Mayerhoefer ME, Lukas J, et al. Rituximab plus bendamustine is active in pretreated patients with extragastric marginal zone B cell lymphoma of the mucosa-associated lymphoid tissue (MALT lymphoma). Ann Hematol 2014;93:249-53. [Crossref] [PubMed]
- Okamura I, Imai H, Mori K, et al. Rituximab monotherapy as a first-line treatment for pulmonary mucosa-associated lymphoid tissue lymphoma. Int J Hematol 2015;101:46-51. [Crossref] [PubMed]
- Raderer M, Wohrer S, Streubel B, et al. Activity of rituximab plus cyclophosphamide, doxorubicin/mitoxantrone, vincristine and prednisone in patients with relapsed MALT lymphoma. Oncology 2006;70:411-7. [Crossref] [PubMed]
- Salar A, Domingo-Domenech E, Estany C, et al. Combination therapy with rituximab and intravenous or oral fludarabine in the first-line, systemic treatment of patients with extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type. Cancer 2009;115:5210-7. [Crossref] [PubMed]
- Joly-Battaglini A, Hammarstrom C, Stankovic B, et al. Rituximab efficiently depletes B cells in lung tumors and normal lung tissue. F1000Res 2016;5:38. [Crossref] [PubMed]
- Orciuolo E, Buda G, Sordi E, et al. 2CdA chemotherapy and rituximab in the treatment of marginal zone lymphoma. Leuk Res 2010;34:184-9. [Crossref] [PubMed]
- Ahmed S, Kussick SJ, Siddiqui AK, et al. Bronchial-associated lymphoid tissue lymphoma: a clinical study of a rare disease. Eur J Cancer 2004;40:1320-6. [Crossref] [PubMed]
- Wislez M, Cadranel J, Antoine M, et al. Lymphoma of pulmonary mucosa-associated lymphoid tissue: CT scan findings and pathological correlations. Eur Respir J 1999;14:423-9. [Crossref] [PubMed]