Surgical approaches of endobronchial neoplasms
Introduction
Pure endobronchial neoplasm, defined as the tumor involving the bronchial lumen mainly, is rare and presents as diverse pathological distributions (1,2). Malignant diseases are more common than benign ones and mostly originate from the surface epithelium. Squamous cell carcinoma (SCC) has recently been replaced by adenocarcinoma as the most common lung cancer. However, SCC remains the most predominant malignant endobronchial histology in adults. In contrast, carcinoid cancer constitutes the majority of endobronchial tumors in children. Mucoepidermoid carcinoma (MEC) and adenoid cystic carcinoma (ACC) are two most common endobronchial cancers derived from submucosal gland. In our experience, MEC is more common than ACC in the bronchus, and benign endobronchial neoplasm is extremely rare (Table 1).
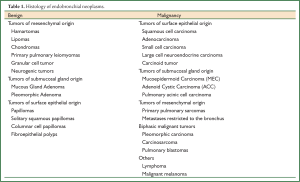
Full Table
Most patients with endobronchial tumors present with symptoms related to cough, chest pain, wheezing, hemoptysis, recurrent pneumonia and weight loss. Endobronchial neoplasms are often diagnosed earlier than tracheal lesions for their smaller diameter. The presence of hemoptysis and obstructive pneumonia indicates possible airway intra-luminal involvement by the tumor and the need for further bronchoscopic and imaging evaluation.
Parenchymal sparing surgery remains a clinical challenge, and is attempted mostly in endobronchial neoplasm treatment (3-6). However, simple segmental bronchial resection is not adequate to achieve curable resection in most malignant endobronchial tumors, especially in patients with lymph node metastasis (7). Carina reconstruction and sleeve lobe resection are often adopted in our patients with primary main bronchial malignancies (8-12). The lesions located in the lobe bronchus are usually treated as a lung tumor with lobectomy or sub lobectomy. More complex bronchial reconstruction can be tried in patients with benign and low-grade malignant tumors if expertise in management is available (13-15). Normal lung should be preserved in patients with damaged cardiopulmonary reserve. Interventional pulmonary therapy is helpful to release airway obstruction in patients with advanced malignancy as a salvage procedure. Therapeutic bronchoscopy has also been employed in children with benign or low-grade malignancy to prevent late anastomotic stenosis from surgical sutures (16). Definitive radiation therapy, including brachytherapy, can be used for patients with localized malignancy and those who refuse to accept surgical treatment. However, any interventional or radiation therapy could increase the difficulty when delayed operation is performed and add more risk of surgery-related complications.
Surgical approaches
Patient selection
It is possible to make parenchymal sparing resections rather than lobectomy or pneumonectomy for the treatment of endobronchial neoplasms with special anatomic locations. However, the selection of a specific surgical option should be determined by clinical factors.
The first is the histology status and pathologic staging. It is possible to preserve the normal lung in patients with benign tumors or low-grade malignancies, and a safe margin can be achieved in 2-4 mm. However, in patients with malignant lesions, especially in those with squamous cell lung cancer, segmental or limited bronchial resection should be carried out cautiously, and a longer surgical margins more than 5mm is needed. On the other hand, in patients with bronchial lung cancer and positive lymph nodes adjacent to the bronchus, only segmental bronchial or lobar sleeve resection could predict early recurrence and poor long-term survival as compared with pneumonectomy (17).
The second is the pulmonary function. Predicted postoperative forced expiratory volume in 1 second (FEV1) indicates a conservative surgical strategy, such as segmental or sleeve resection rather than simple pneumonectomy or lobectomy. However, more complex surgical plasty could easily lead to postoperative atelectasis, which is more likely to occur in cases of unmatched anastomosis. Although most strictures from edema could be released spontaneously, they should be managed carefully in patients with marginal respiratory reserve. Some patients with endobronchial tumors may suffer complete atelectasis distal to the diseased airway preoperatively. Lung perfusion of the impaired side can be restored after the airway is reopened with bronchial sleeve resection. For those patients, this “nascent” pulmonary function should be considered in calculating the postoperative predicted FEV1 (18,19).
Meanwhile, preoperative assessment of the local lesion is the key point to tailor the surgical plan. The surgeon should be at hand or do it personally when the diagnostic bronchoscopy is carried out. Accurate and precise location should include each end of the margin, and the spatial relationships with the surrounding bronchial airways. In this aspect, fluorescence bronchoscopy could provide more information for potential submucosal invasion or minimal mucosal change. Bronchoscopy should be scheduled again at the time of operation to confirm the previous finding. Given the limited length of the bronchus, any preoperative endobronchal intervention could kiss goodbye to the opportunity for successful reconstruction. High resolution CT scan can reveal the detailed information of the bronchus and the lymph node status. PET/CT can show the involvement of regional and distal metastasis. However, any possible lymph node metastasis in mediastinum should be confirmed histologically by mediastinoscopy or EBUS. The 11th-13th lymph nodes can be accessed by mini-probe-ultrasound-guided needle aspiration.
The requirements on systemic conditions are not significantly different from other pulmonary operations. Long-term use of high-dose steroids is contraindicated to bronchial reconstruction. Pulmonary infection with fever and elevated white blood cell should be treated preoperatively. However, obstructive atelectasis is not a contraindication, because this condition can be converted immediately after the diseased airway is corrected with careful postoperative care. Active tuberculosis (TB) should be treated medically for at least 6 months before resection.
As most bronchial resections and reconstructions involve the conditions of open airway, selected single lung ventilation is mandatory. Double-lumen endobronchial intubation can satisfy most of the conditions. However, for patients with difficult airways and pediatric patients, a more complex approach is necessary.
Evaluation
Careful preoperative evaluation is very important for a tailored operation design. Bronchoscopy is a necessity in all cases to get pathologic results and define the range of tumor involvement. No indication is available for interventional resection if the curable surgical excision is planed shortly. The use of autofluorescence-reflectance bronchoscopy as an adjunct to white light bronchoscopy can improve the detection and localization of intraepitheal neoplasia and invasion (20,21). CT scan is a key tool to evaluate the lymph node status and extra-bronchial invasion. Any suspected metastasis should be evidenced by biopsy with EBUS or mediastinoscopy. Currently, PET can add more information to occult lymph node metastasis. Tri-dimensional reconstruction with CT scan is helpful to set up a more straight understanding of the disease.
Surgical technique
This section concentrates on the bronchial and tracheal reconstruction for bronchus-original neoplasms rather than regular lobectomy and pneumonectomy.
The standard surgical approach for bronchial disease is through posterolateral thoracotomy. If the proximal end of the left main bronchus or carina is involved, sternotomy, bilateral thoracotomy, or clamshell incision could be selected for better exposure. With the availability of VATS pulmonary surgery, some centers have attempted to perform bronchial plasty via a minimally invasive approach. However, this new technique is limited for the right upper lobe sleeve resection in most cases.
If the bronchus/lobe sleeve resection is planned, extensive lymph node sampling should be performed to confirm the absence of local regional metastasis. The evaluated range should include any accessible area of the isolateral lung and mediastinum. A detailed procedure requires sampling and intraoperative frozen sections of abnormal and representative lymph nodes from the fissure and along the bronchus of the lobe to be preserved. Any metastasis along the bronchus or located at the fissure indicates a more extensive lung resection rather than bronchial sleeve resection along.
Isolation of the bronchus on the right side is easier than that on the left side. If a malignant lesion involves the right upper lobe bronchial orifice, exploration should be extended to the distal end of the intermittent bronchus and carina. Hilar release and pre-tracheal dissection is useful for the condition of carina reconstruction. Exposure of the left main bronchus, especially the proximal end, is usually difficult. Isolation of the aortic arch can increase more space for this area. Sometimes, we prefer to sacrifice the left superior segment to provide additional space for complex left main bronchial reconstruction, and the outcome is excellent.
When the bronchus is isolated and ventilation is controlled, the bronchus can be divided. In neoplastic disease, frozen section analysis is obtained by sending a thin ring of tissue from the two ends of airway to be anastomosed. A safe margin more than 5 mm is needed for the malignant tumor, and it can be compromised to 2 mm for the low-grade and benign lesions.
Currently, no attempt is made to tailor either end of the bronchus to correct the unmatched orifice in the case of end-to-end anastomosis. A scuff anastomosis can provide a patent and complete connection for such a kind of airway reconstruction. Carinal involvement is common for the right main bronchial malignancy. Various reconstruction methods have been introduced for half carina resection. Miyamoto’s technique is an excellent alternative to be selected in this condition (22). When the proximal end of the left main bronchus is invaded, left main bronchial sleeve resection and carinal reconstruction can be performed to secure a free margin. Several approaches for the left part of carinal operation have been reported, such as midline sternotomy, left thoracotomy, bilateral thoracotomy, midline sternotomy with left thoracotomy, and clamshell thoracotomy (23,24). However, left side carinal reconstruction is much more difficult than right side carinal reconstruction due to the narrow operative field and minimal mobilization of the airway. Excellent exposure and reliable ventilation must be guaranteed no matter what approach is selected. Modified one-stoma-type carinoplasty could help avoid aggressive carinal resection for lung saving, and the outcome has proved to be good. To make more space and minimize tension under the aortic arch, sacrificing a lobe can be performed as an alternative to pneumonectomy.
For tumors involving the distal end of the main bronchus, upper bronchus, right intermediate and superior segment of the lower lobe, finely tailored bronchoplasy is demanded (25,26). When lobar and/or segmental sleeve resection with reconstruction is performed, sacrificing one adjacent segment could leave more space to do anastomosis.
Selection of the anastomotic technique depends on the surgeon’s favor. Continuous suture with 4-0 or 3-0 PDS can save more time and achieve comparable results as compared with interrupted suture with 4-0 Vicryl (27,28). All anastomoses must be evaluated by bronchoscopy. The inflation test is also important to test the patency of the distal lung. Immediate collapse is more important to infer a patent anastomosis than enforced inflation. Any unsatisfactory anastomosis should be corrected immediately, even when conversion to lobectomy or pneumonectomy is necessary.
Autologous tissue wrapping is not routinely used for any kind of airway plasty. It is only indicated as a salvage procedure when the anastomosis is not satisfactory. Pericardial fat pad (PFP) sounds a good candidate for easy harvest and minimal invasion. However, in our experience, fat liquefaction is the inevitable fate of PFP with a risk of local infection. The intercostal muscle remains the optimal coverage tissue for airway anastomosis.
Postoperative care
The postoperative course after endobronchial tumor resection is usually as even as regular lung surgery. Pain management is the key point to guarantee effective cough and physical therapy. Any sign of atelectasis distal to the anastomosis should be evaluated by bronchoscopy if the condition permits. Temporary anastomosis edema can be managed conservatively (29). Re-surgery and interventional stent therapy should be considered when surgical-related stenosis occurs.
Perioperative outcome and long-term survival
There is no consistent result with respect to the operative mortality and morbidity in surgical treatment of endobronchial neoplasm because of the low incidence of the condition. If the carina is involved, especially at the left side, the incidence of surgical complications will be higher than other locations. Anastomotic stenosis is the most common complication after bronchial plasty including early and late strictures. Late stenosis is usually treated by interventional bronchoscopic therapy. The airway should be evaluated with careful bronchoscopic examination. A personalized design is mandatory for any interventional therapy.
Long-term survival is different depending on the histology. For low-grade malignant and benign lesions, complete resection can achieve a very good survival outcome. The lymph node status and complete resection will generate different survival outcomes for malignant tumors, especially for SCC. Deliberate parenchyma-sparing surgery with misdiagnosis of metastasis could be harmful to the patients.
Conclusions
Endobronchial neoplasm is rare with a histological diversity. A personalized therapeutic strategy is important to maximize the efficacy of therapy. The lymph node status should be evaluated thoroughly before parenchyma-sparing surgery for malignant endobronchial tumors. Do not hesitate to do a lobectomy or pneumonectomy in cases with positive intrapulmonary lymph nodes. Carinal reconstruction should be performed skillfully to get a negative proximal margin whenever needed. Sacrificing a segment or lobe to obtain a wider surgical field may spare pneumonectomy in some cases.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Wilson RW, Kirejczyk W. Pathological and radiological correlation of endobronchial neoplasms: Part I, Benign tumors. Ann Diagn Pathol 1997;1:31-46. [PubMed]
- Wilson RW, Frazier AA. Pathological and radiological correlation of endobronchial neoplasms: Part II, Malignant tumors. Ann Diagn Pathol 1998;2:31-4. [PubMed]
- Bölükbas S, Schirren J. Parenchyma-sparing bronchial sleeve resections in trauma, benign and malign diseases. Thorac Cardiovasc Surg 2010;58:32-7. [PubMed]
- Bagan P, Le Pimpec-Barthes F, Badia A, et al. Bronchial sleeve resections: lung function resurrecting procedure. Eur J Cardiothorac Surg 2008;34:484-7. [PubMed]
- Ragusa M, Vannucci J, Cagini L, et al. Left main bronchus resection and reconstruction. A single institution experience. J Cardiothorac Surg 2012;7:29. [PubMed]
- Cerfolio RJ, Deschamps C, Allen MS, et al. Mainstem bronchial sleeve resection with pulmonary preservation. Ann Thorac Surg 1996;61:1458-62; discussion 1462-3. [PubMed]
- Kim YT, Kang CH, Sung SW, et al. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg 2005;79:1153-61; discussion 1153-61. [PubMed]
- Yamamoto K, Miyamoto Y, Ohsumi A, et al. Surgical results of carinal reconstruction: an alterative technique for tumors involving the tracheal carina. Ann Thorac Surg 2007;84:216-20. [PubMed]
- Tsakiridis K, Visouli AN, Zarogoulidis P, et al. Early hemi-diaphragmatic plication through a video assisted mini-thoracotomy in postcardiotomy phrenic nerve paresis. J Thorac Dis 2012;4:56-68. [PubMed]
- Tsakiridis K, Darwiche K, Visouli AN, et al. Management of complex benign post-tracheostomy tracheal stenosis with bronchoscopic insertion of silicon tracheal stents, in patients with failed or contraindicated surgical reconstruction of trachea. J Thorac Dis 2012;4:32-40. [PubMed]
- Tsakiridis K, Visouli AN, Zarogoulidis P, et al. Lost in time pulmonary metastases of renal cell carcinoma: complete surgical resection of metachronous metastases, 18 and 15 years after nephrectomy. J Thorac Dis 2012;4:69-73. [PubMed]
- Tsakiridis K, Visouli AN, Zarogoulidis P, et al. Resection of a giant bilateral retrovascular intrathoracic goiter causing severe upper airway obstruction, 2 years after subtotal thyroidectomy: a case report and review of the literature. J Thorac Dis 2012;4:41-8. [PubMed]
- Jiang X, Dong X, Zhao X, et al. Bronchial sleeve resection distal to the main bronchus with complete pulmonary preservation for benign or low-grade malignant tumors. Ann Thorac Surg 2007;84:e19-21. [PubMed]
- Yavuzer S, Yüksel C, Kutlay H. Segmental bronchial sleeve resection: preserving all lung parenchyma for benign/low-grade neoplasms. Ann Thorac Surg 2010;89:1737-43. [PubMed]
- Nowak K, Karenovics W, Nicholson AG, et al. Pure bronchoplastic resections of the bronchus without pulmonary resection for endobronchial carcinoid tumours. Interact Cardiovasc Thorac Surg 2013. [Epub ahead of print]. [PubMed]
- Brokx HA, Risse EK, Paul MA, et al. Initial bronchoscopic treatment for patients with intraluminal bronchial carcinoids. J Thorac Cardiovasc Surg 2007;133:973-8. [PubMed]
- Fadel E, Yildizeli B, Chapelier AR, et al. Sleeve lobectomy for bronchogenic cancers: factors affecting survival. Ann Thorac Surg 2002;74:851-8; discussion 858-9. [PubMed]
- Bagan P, Le Pimpec-Barthes F, Badia A, et al. Bronchial sleeve resections: lung function resurrecting procedure. Eur J Cardiothorac Surg 2008;34:484-7. [PubMed]
- Takahashi S, Hata Y, Sasamoto S, et al. Recovery of lung perfusion after sleeve resection for tuberculous bronchial stenosis. Ann Thorac Surg 2012;93:2041-3. [PubMed]
- Edell E, Lam S, Pass H, et al. Detection and localization of intraepithelial neoplasia and invasive carcinoma using fluorescence-reflectance bronchoscopy: an international, multicenter clinical trial. J Thorac Oncol 2009;4:49-54. [PubMed]
- Visouli AN, Darwiche K, Kourtoglou GI, et al. Primary lung carcinoid, a rare cause of paraparesis: report of a case and review of the literature. J Thorac Dis 2012;4:49-55. [PubMed]
- Sayar A, Solak O, Metin M, et al. Carinal resection and reconstruction for respiratory tumors using Miyamoto’s technique. Gen Thorac Cardiovasc Surg 2012;60:90-6. [PubMed]
- Miyazaki T, Yamasaki N, Tsuchiya T, et al. Left sleeve pneumonectomy via a clamshell incision for lung cancer with carinal invasion: report of a case. Surg Today 2012;42:593-6. [PubMed]
- Kondo T, Sagawa M, Sato M, et al. Left sleeve pneumonectomy performed through a clamshell incision with extracorporeal membrane oxygenation for bronchogenic carcinoma: report of two cases. Surg Today 1999;29:807-10. [PubMed]
- Jiang X, Dong X, Zhao X, et al. Bronchial sleeve resection distal to the main bronchus with complete pulmonary preservation for benign or low-grade malignant tumors. Ann Thorac Surg 2007;84:e19-21. [PubMed]
- Tsubota N. Bronchoplasty at the level of the segmental bronchus. Semin Thorac Cardiovasc Surg 2006;18:96-103. [PubMed]
- Yu JA, Weyant MJ. Techniques of bronchial sleeve resection. Semin Cardiothorac Vasc Anesth 2012;16:196-202. [PubMed]
- Di Rienzo G, Go T, Macchiarini P. Simplified anastomotic technique for end-to-side bronchial reimplantation onto the trachea or contralateral main bronchus after complex tracheobronchial resections. J Thorac Cardiovasc Surg 2002;124:632-5. [PubMed]
- Porpodis K, Karanikas M, Zarogoulidis P, et al. A case of typical pulmonary carcinoid tumor treated with bronchoscopic therapy followed by lobectomy. J Multidiscip Healthc 2012;5:47-51. [PubMed]


