Viscoelastic testing inside and beyond the operating room
Background
Hemorrhage in the perioperative period is a significant cause of patient morbidity and mortality after major surgery (1,2). An estimated one-third of post-surgical bleeding is the result of non-surgical, medical coagulopathy (3). Coagulopathy further increases risk of bleeding complications and requires both timely diagnosis as well as correction (4). Perioperative coagulopathy is frequently multifactorial, including dilution of plasma volume with intravenous fluid or packed red blood cells (pRBC), consumption of coagulation factors from ongoing bleeding, administration of antithrombotic medications such as heparin or antiplatelet agents, or patient-specific conditions such as end-stage liver disease, or inherited factor deficiencies.
The treatment for perioperative bleeding consists of the transfusion of blood products. Anemia is corrected by means of transfusion of pRBC and treatment may be guided by patient hemoglobin or hematocrit levels, clinical symptoms, or elected empirically in settings of active hemorrhage. The correction of coagulopathy entails primarily the use of thawed plasma, cryoprecipitate, or platelets. Plasma contains all plasma coagulation factors. Cryoprecipitate is prepared from plasma, and contains fibrinogen, factor VIII, von Willebrand factor, factor XIII, and fibronectin, in a small infusion volume. Platelets are transfused to correct both quantitative deficiencies as well as qualitative platelet defects in patients (5). Additional factor concentrates more recently approved for use in the US include concentrated fibrinogen, prothrombin complex concentrate (PCC), and recombinant factor VIIa (6).
Blood products are valuable resources, because of both scarcity and cost, and carry not-insignificant risks of transfusion-related morbidity, such as transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload (TACO), viral or bacterial infection, and recipient immunomodulation (7,8). As such, the decision to transfuse should be made judiciously using both clinical and laboratory data. In situations of massive hemorrhage and shock, such as trauma, transfusion and blood component therapy may be prescribed empirically. Whenever possible, laboratory data should guide the use of blood products.
A complete blood count (CBC) can assess hemoglobin, hematocrit, and platelet count, and various point-of-care devices in clinical use can approximate values with reasonable accuracy (9,10). Traditionally, to assess coagulopathy, the prothrombin time (PT) and international normalized ratio (INR) were used to assess the extrinsic and common pathways, while the activated partial thromboplastin time (aPTT) was used to assess the intrinsic and common pathways. A laboratory fibrinogen level may supplement these basic coagulation tests. However, these tests have a variety of limitations including laboratory turnover times, lack of specificity regarding the quality of the clot formed or fibrinolysis and inability to predict risk of bleeding or clotting ability as the tests are performed in the absence of platelets and other blood components in a standardized environment (11,12). More recently, viscoelastic point-of-care testing has shown promise in diagnosing perioperative coagulopathy and targeting blood products to specific clot defects.
Viscoelastic testing
Viscoelastic testing in general refers to several commercially available point-of-care tests that use a sample of patient blood to derive various parameters pertaining to the quality of clot formed. The conceptual technology was invented in 1948 (13), but clinical use was not adopted until the 1980s (14). The analyzer imitates sluggish venous blood flow and derives measurements of the kinetics of each stage of clot initiation, strength, and lysis. A small sample of patient blood is placed into a cup, and a sensor rod is inserted into the blood sample. Either the cup or the rod is then gently rotated, with a subsequent clot forming between the cup and rod. The change in speed and pattern of change are measured by a computer and depicted as a graph. In thromboelastography (TEG), the cup rotates, while in rotational thromboelastometry (ROTEM) the sensor rod rotates (11,15).
Viscoelastic testing has several distinct advantages over traditional coagulation assays. The point-of-care nature of the tests yields results quickly. The results are displayed in both graphical format as well as various numerical measurements, with reference ranges, which can aid rapid diagnosis of specific coagulopathies. They may be performed at various temperatures, ranging from 22 to 42 °C, to demonstrate the effects of acidosis, and hyperthermia or hypothermia on coagulation. Probably most importantly, the tests are able to detect specific defects in coagulation, such as hypofibrinogenemia, hyperfibrinolysis, factor deficiency, and heparin effect (16). These advantages allow rapid diagnosis of specific coagulopathies, and therefore suggest specific treatments, including blood products and medications, which may reduce overall transfusion requirement, decrease hemorrhage, and decrease mortality (17).
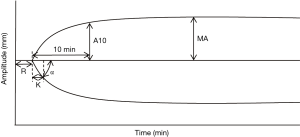
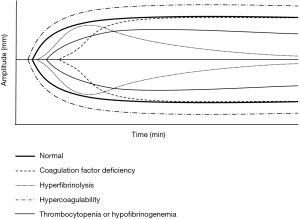
The two predominant commercially-available systems of viscoelastic testing are TEG and ROTEM. A third device, Sonoclot, has been studied less. There are several differences in the assays available on TEG versus ROTEM, but there has been no evidence suggesting clinical superiority of one system over another (18). The classical TEG uses a graphical display to show the initiation, strengthening, and ultimately lysis of clot, and measures a number of variables related to the graphic: reaction time (R), the time to clot initiation; kinetics time (K), the time to reach a certain threshold of clot strength; alpha (α) angle, slope between R and K; maximum amplitude (MA), the maximum strength of the clot; A30, the strength of the clot at 30 minutes; LY30, the degree of thrombolysis at 30 minutes (19) (Figure 1). Abnormalities of any of these variables suggest specific coagulopathies (Figure 2). For example, a prolonged R time suggests deficiency of clotting factors and may be treated with transfusion of plasma. However, some abnormalities, including decreased α or MA result from deficiencies in either platelets or fibrinogen, and TEG is unable to differentiate between the two (14).
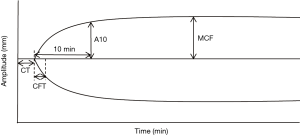
ROTEM uses an almost-identical graphical results display compared to TEG, and also measures similar parameters on the graphic, but uses different names (Figure 3). R time is clotting time (CT), K time is clot formation time (CFT), MA is maximum clot firmness (MCF), and LY30 is clot lysis (CL30). Of note, while the graphics and measurements of TEG and ROTEM are akin conceptually, because of test reagent differences, their values cannot be directly compared. The ROTEM system also markets multiple assays for analysis of various aspects of the coagulation cascade. These include EXTEM, for evaluating the extrinsic pathway, INTEM, for the intrinsic pathway, FIBTEM, for evaluation of fibrinogen contribution to clot formation, and HEPTEM and APTEM for evaluation of heparin effect or thrombolysis reversal. Of these assays, FIBTEM and EXTEM used in conjunction can differentiate hypofibrinogenemia and thrombocytopenia. FIBTEM A10 (clot strength at 10 minutes) has been found to correlate to serum fibrinogen levels (20), and also correlates to FIBTEM MCF (21-23). Thus when FIBTEM MCF or A10 is low, hypofibrinogenemia is likely and may be treated with cryoprecipitate or fibrinogen concentrate (18). Once FIBTEM MCF or A10 has been corrected, the EXTEM MCF or A10 can be analyzed, and if found to be low, suggests thrombocytopenia as the cause of coagulopathy. HEPTEM and INTEM can be used together to demonstrate heparin-induced coagulopathy, as a prolonged CT on the INTEM due to heparin effect will normalize on the HEPTEM due to the addition of heparinase in the assay. Similarly, a comparison of EXTEM and APTEM is used to diagnose fibrinolysis—if a fibrinolytic pattern is seen on EXTEM, the aprotinin in APTEM should reverse the abnormality. Thus, using multiple assays on ROTEM may yield more specific diagnoses in coagulopathy compared to a classic TEG test.
Clinical outcomes of viscoelastic testing
There have been numerous studies published on the clinical use of TEG and ROTEM. A majority of studies have been in perioperative cardiac surgery patients, while many have considered TEG or ROTEM use in other cases at risk for hemorrhage including liver transplantation, trauma, and orthopedic surgery. Significant heterogeneity exists in these studies, many of which are observational in nature, though some randomized-controlled trials (RCTs) exist. A Cochrane review was published initially in 2011 and updated in 2016, which analyzes the literature to compare TEG/ROTEM-guided transfusion management against conventional transfusion management (17). The review searched the literature for only RCTs comparing transfusions guided by viscoelastic testing and transfusions guided by either clinical judgement, laboratory data, or a combination of both. The updated review included a total of 17 studies (1,493 patients), of which 2 included only pediatric patients, 1 was in the setting of liver transplantation, 1 in setting of wound excisions of burn patients, while the majority of studies were in the cardiac surgery setting (1,435 patients; 96% of patients).
The review concluded that TEG or ROTEM-guided transfusion practice appeared to reduce overall mortality [7.4% versus 3.9%, risk ratio (RR) 0.52, 95% confidence interval (CI), 0.28–0.95], pooling the results of eight studies and 717 patients. In TEG/ROTEM-guided transfusion management groups compared to transfusion management guided by any other method, there was also a statistically significant decrease in the proportion of patients transfused with pRBC (RR 0.86, 95% CI: 0.79–0.94, 10 studies, 832 patients), plasma (0.57, 95% CI: 0.33–0.96, 8 studies, 761 patients), platelets (RR 0.73, 95% CI: 0.60–0.88, 10 studies, 832 patients), overall plasma or platelet transfusion for hemostasis, and fewer patients with dialysis-dependent renal failure. No differences were found in the proportion of patients needing surgical re-intervention, massive transfusion, or patients with excessive bleeding. The reviewers, however, judged the quality of evidence for all of these conclusions to be low. Only two studies were deemed to be at low risk of bias, and overall there was large heterogeneity, low number of events, imprecision, and indirectness in the included studies. It would therefore appear that in a largely cardiac surgery patient population, while TEG/ROTEM-guided transfusion strategies might decrease transfusion requirements of pRBC, plasma, and platelets, with a tendency towards improved mortality outcomes, conclusive recommendations could not be made due to limitations of study design and power.
While the Wikkelsø paper addresses the comparison between TEG and ROTEM to guide transfusions to conventional transfusion practices, its scope was primarily confined to elective cardiac surgery patients at low-to-moderate risk of bleeding. Trauma patients are at high risk of hemorrhage-related morbidity and mortality, and trauma-induced coagulopathy (TIC) further increases the risk of complications, need for transfusion, and length of stay (24). Previous studies in the trauma patient population have demonstrated hypocoagulability on viscoelastic tests to predict the need for massive transfusion (25-28), the need for transfusion in general (29), and coagulopathy-related mortality (27,30).
A Cochrane review examined the literature on the use of TEG and ROTEM for diagnosing TIC (31). The review included cross-sectional studies as well as case-control studies that used TEG or ROTEM to diagnose TIC, compared to the conventional studies PT ratio of 1.2 or greater, or INR of 1.5 or greater. Only three studies were included in the final analysis. All three used ROTEM, and used EXTEM clot amplitude (CA) at 5, 10, or 15 minutes to diagnose TIC. The studies were conducted in the UK, France, and Afghanistan in both civilian and military settings. Given the small number of studies and study heterogeneity, a meta-analysis could not be performed, and instead qualitative results were given. For CA5, sensitivity of ROTEM for TIC was 70%, specificity 86% for one study; sensitivity 96% and specificity 58% for another study. For CA10, sensitivity was 100%, specificity 70%. For CA15 sensitivity was 88%, specificity 100%. The risk of biases for all studies was high, suggesting limited applicability and validity of the findings. Overall, there was very low quality evidence for using ROTEM to diagnose TIC. No studies used TEG.
Da Luz et al. (32) searched the literature for changes in outcome in the trauma patient population with the use of viscoelastic testing. This descriptive systematic review included all observational studies as well as RCTs which used TEG or ROTEM in adult trauma patients, and ultimately included 55 studies (12,489 patients). Only 3 of 47 studies had low risk of bias, while 37 of 47 studies had low concerns regarding applicability. Additionally, standard measures of diagnostic accuracy were inconsistently reported across studies. Many TEG/ROTEM measurements were associated with early coagulopathies, and many abnormalities predicted the need for massive transfusion and death, but the predictive performance was not consistently superior to conventional coagulation tests. Overall, the review concluded that observational data provides some limited evidence that TEG/ROTEM can diagnose early trauma coagulopathy and may predict blood product transfusion and mortality, though these results as well as effects on other outcomes are unproven in randomized trials.
Another systematic review evaluated the cost-effectiveness of using viscoelastic testing compared to conventional coagulation tests (33) in the setting of cardiac surgery patients, trauma patients with TIC, and women with post-partum hemorrhage. Thirty-nine studies based on 31 studies were included in the review for clinical outcome differences. The results from the studies were heterogeneous and could not be pooled for meta-analysis; they were, therefore, reported qualitatively. The review was unable to find relevant studies on viscoelastic testing and post-partum hemorrhage, and could not find sufficient evidence on outcome measures for the TIC patient population. For the cardiac surgery patient population, 11 RCTs overall showed significant reduction in pRBC, plasma, and platelet transfusions with viscoelastic testing. Other outcomes such as factor VIIa, PCC, or fibrinogen transfusions, surgical reintervention, length of hospital stay, and mortality did not appear to differ between viscoelastic testing and conventional coagulation testing groups, though these outcomes were not reported in most studies and those that did lacked statistical power. The viscoelastic group appeared to experience reduced bleeding and shorter intensive care unit (ICU) stay, but this outcome was inconsistently reported across studies. The studies were all performed on TEG or ROTEM, none on Sonoclot, and no apparent differences between devices were seen.
Regarding the cost-effectiveness of viscoelastic testing in cardiac surgery patients, the review concluded that it is cost-saving and more effective than conventional coagulation tests, owing mostly to the decrease in blood products transfused. The per-patient cost-savings were £43 for ROTEM, £79 for TEG, and £132 for Sonoclot, as a result of the cost of purchasing each base viscoelastic system, with the basic ROTEM system being most expensive, and Sonoclot the least expensive. When alternative assay combinations were modelled, the TEG system could become more expensive than ROTEM. Of note, as no Sonoclot studies were found for the clinical outcomes portion of the review, the system was assumed to be equally efficacious clinically as TEG or ROTEM for the purposes of the cost-effectiveness analysis. In models, viscoelastic testing was no longer cost-saving when the number of tests performed per machine per year was less than 326. In the TIC patient population, viscoelastic testing was even more cost-saving. The per-patient savings were £688 for ROTEM, £721 for TEG, and £818 for Sonoclot. The increased savings were due to the higher volume of blood products transfused in trauma patients. This conclusion is predicated on the assumption that viscoelastic testing leads to improved clinical outcomes in the trauma population, which again could not be established from existing studies, and in the absence of convincing data, the cost-savings remain more theoretical.
Broader applications of viscoelastic testing
While viscoelastic testing is currently primarily used to direct hemostatic management in the bleeding patient, the broader applications of viscoelastic testing has been investigated in smaller trials (34). Both the diagnostic and the prognostic role of viscoelastic testing are currently being studied in a range of clinical areas.
In cirrhotic patients with severe coagulopathy, one RCT demonstrated greatly decreased use of blood products when using a TEG-guided transfusion strategy prior to invasive procedures compared to the standard of care, without an increase in bleeding events (35), in an echo of aforementioned findings from the cardiac surgery setting.
In sepsis, viscoelastic testing identifies alterations in the coagulation system that contribute to organ system dysfunction (36). An observational study by Brenner et al. (37) showed that septic patients with disseminated intravascular coagulation (DIC) have hypocoagulable profiles, whereas septic patients without DIC have hypercoagulable profiles. The authors suggest that thromboelastometry could be used as an early diagnostic test for identification of septic patients at high risk of developing DIC. A cohort study by Adamzik et al. (38) suggests that thromboelastometry may predict 30-day survival better than validated scoring systems, such as the Simplified Acute Physiology Score II and the Sequential Organ Failure Assessment. A study by Ostrowski et al. (39) echoed the prognostic power of thromboelastometry in sepsis by showing that a hypocoagulable MA independently predicted 28-day mortality. Viscoelastic testing thus has the potential to aid in the early diagnosis of sepsis and to risk stratify septic patients.
Obstetric hemorrhage poses a unique resuscitative challenge. Karlsson et al. (40) suggested that TEG may improve hemostasis when estimated blood loss is greater than two liters in maternal obstetric hemorrhage due to rapidity of results. A prospective observational study by Collins et al. (41) found FIBTEM to be an independent predictor for progression to obstetric hemorrhage greater than 2,500 cc. Although it deserves further investigation, there is not currently enough evidence to substantiate an evidence-based recommendation for the use of viscoelastic testing to guide the management of obstetric hemorrhage (42).
Hypercoagulable states offer yet another opportunity to utilize viscoelastic testing. Post-surgical patients at risk for thromboembolic events may benefit from pre-operative viscoelastic testing for risk stratification. In a prospective, observational study, Rafiq et al. (43) found that hypercoagulability identified by TEG prior to coronary artery bypass grafting correlated with a higher risk for a combination of thromboembolic complications and death after surgery. Similarly, Hincker et al. (44) also showed that pre-operative ROTEM may detect patients at increased risk for postoperative thromboembolic complications after major non-cardiac surgery. The relationship between hypercoagulability and thromboembolic events in other patient populations, such as oncologic patients and trauma patients, is less clear (45,46).
Direct oral anticoagulants (DOACs), also previously referred to as novel oral anticoagulants (NOACs), are a group of anticoagulants which directly inhibit factor Xa (rivaroxaban, apixaban, edoxaban), or inhibit thrombin (dabigatran). These agents are used increasingly for long-term anticoagulation to treat or prevent deep venous thromboses, pulmonary emboli, or for stroke prophylaxis in atrial fibrillation (47). In acute bleeding events in the presence of these agents, viscoelastic testing has been used to aid the diagnosis of coagulopathy. At least two studies have linked dabigatran to abnormalities on ROTEM, including prolonged EXTEM-CT, decreased A10, and decreased FIBTEM (48,49). Additionally, ROTEM EXTEM has been used as an adjunctive test to monitor the effect of dabigatran reversal agent idarucizumab in pigs (50). EXTEM-CT can also be prolonged by therapeutic serum levels of the factor Xa inhibitors rivaroxaban (49) and apixaban (51). INTEM-CT may also be prolonged by rivaroxaban and apixaban, but this was inconsistently shown in an in vitro study (51). Viscoelastic tests are promising in the setting of acute bleeding from DOACs, given their speed of results compared to conventional coagulation tests, but their role is yet to be fully clarified in larger clinical trials.
Inherited bleeding disorders are typically monitored by measuring the concentration of deficient clotting factors. However, the concentration of a deficient clotting factor does not necessarily correlate with the tendency to bleed (52). As a global assay that determines whole blood clot formation and dissolution, viscoelastic testing may be able to more accurately predict bleeding tendency. For example, Sørensen et al. (53) suggested a possible role for viscoelastic testing in guiding perioperative therapy in hemophilia patients. Tran et al. (54) performed a prospective crossover study to assess the capability of ROTEM to tailor the use of bypassing agents in hemophilia A patients with inhibitors. Although the thrombin generation assay was more sensitive to differences in the treatment response, ROTEM performed favorably. More studies are needed to investigate the role of viscoelastic testing in supplementing specific clotting factor tests to better assess bleeding risk.
Extracorporeal membrane oxygenation (ECMO) is a method of replacing pulmonary gas exchange and/or supporting cardiac function in patients with refractory pulmonary or cardiac failure. Patients on ECMO require persistent systemic anticoagulation to avoid circuit clotting and machine failure, typically accomplished with heparin infusion. Viscoelastic tests have been investigated as a method of monitoring the anticoagulation status of ECMO patients (55). One study seeking to characterize the changes on ROTEM during ECMO continuously captured ROTEM results along with conventional coagulation test results for 10 ECMO patients (56). The study showed that EXTEM-CT was low in roughly half of patients, while INTEM-CT was normal in most. MCF was normal or high in the majority of patients, but when low it predicted bleeding events.
Ventricular assist devices (VADs) to support ventricular function in end-stage heart failure also require patients on systemic anticoagulation. Despite INR within goal range, however, thromboembolic complications still occur in these patients (57,58). Viscoelastic tests may also play a role in monitoring anticoagulation for these patients. Two case reports have noted that shortened CT on native ROTEM (NATEM) or increased MCF on ROTEM may predict thromboembolic complications in VAD patients (59,60). A small study by Majeed et al. (61) used TEG results as part of a panel of coagulation tests to detect aspirin hyporesponsiveness to predict thromboembolic events in VAD patients. The study noted that only 1 of 14 thromboembolic events was predicted by aspirin hyporesponsiveness as indicated by TEG, suggesting that aspirin hyporesponsiveness may be unrelated to thromboembolic events.
Conclusions
Hemorrhage is a major contributor to morbidity and mortality during the perioperative period. Current methods of diagnosing coagulopathy have various limitations including laboratory runtimes, lack of information on specific abnormalities of the coagulation cascade, lack of in vivo applicability, and lack of ability to guide the transfusion of blood products. Viscoelastic testing offers a promising solution to many of these problems in its shorter runtime, ability to characterize stages of clot formation and dissolution, and evaluation of coagulation ability as a whole. The two most-studied systems, TEG and ROTEM, offer similar graphical and numerical representations of the initiation, formation, and lysis of clot, with ROTEM able to discriminate between contributions to coagulopathy by hypofibrinogenemia and thrombocytopenia more readily. In systematic reviews on the clinical efficacy of viscoelastic tests, the majority of trials analyzed were in cardiac surgery patients. RCTs exist, but only few are at low risk of bias from a methodological standpoint, and various outcome measures are inconsistently reported, weakening the conclusions to be made regarding the topic.
Reviews of the literature suggest that transfusions of pRBC, plasma, and platelets are all decreased when transfusion decisions were guided by viscoelastic tests rather than by clinical judgement or conventional laboratory tests. Mortality appears to be lower in the viscoelastic testing groups, but this result was underpowered using existing data. Surgical reintervention rates and massive transfusion rates did not seem to differ between groups. Cost-effectiveness studies seem also to favor viscoelastic testing. Assuming higher numbers of tests are run per machine per year, cost-saving might be warranted in cardiac surgery patients as well as trauma patients, although clinical outcomes for superiority of viscoelastic testing is lacking in the latter population. In models, ROTEM is the most expensive system and yields less savings compared to TEG or Sonoclot, but this finding may change depending on the specific combination of assays purchased for each system. Viscoelastic testing has also been investigated in small studies in other arenas, such as sepsis, obstetric hemorrhage, inherited bleeding disorders, perioperative thromboembolism, and management of anticoagulation for patients on mechanical circulatory support systems or DOACs. While results are intriguing, no large trials have taken place to date. Viscoelastic testing remains a relatively novel method of assessment of coagulation ability, and evidence for its use appears favorable in reducing blood product transfusions, especially in cardiac surgery patients. Undoubtedly, future studies will further elucidate its impact on patient outcomes and inform its use in different patient populations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 2004;44:1453-62. [Crossref] [PubMed]
- Clifford L, Jia Q, Yadav H, et al. Characterizing the Epidemiology of Perioperative Transfusion-associated Circulatory Overload. Anesthesiology 2015;122:21-8. [Crossref] [PubMed]
- Hall TS, Brevetti GR, Skoultchi AJ, et al. Re-exploration for hemorrhage following open heart surgery differentiation on the causes of bleeding and the impact on patient outcomes. Ann Thorac Cardiovasc Surg 2001;7:352-7. [PubMed]
- Hardy JF, de Moerloose P, Samama CM. The coagulopathy of massive transfusion. Vox Sang 2005;89:123-7. [Crossref] [PubMed]
- Corredor C, Wasowicz M, Karkouti K, et al. The role of point-of-care platelet function testing in predicting postoperative bleeding following cardiac surgery: a systematic review and meta-analysis. Anaesthesia 2015;70:715-31. [Crossref] [PubMed]
- Lee FM, Chan AK, Lau KK, et al. Reversal of New, Factor-specific Oral Anticoagulants by rFVIIa, Prothrombin Complex Concentrate and Activated Prothrombin Complex Concentrate: A Review of Animal and Human Studies. Thromb Res 2014;133:705-13. [Crossref] [PubMed]
- Delaney M, Wendel S, Bercovitz RS, et al. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825-36. [Crossref] [PubMed]
- Muszynski JA, Spinella PC, Cholette JM, et al. Transfusion-related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion 2017;57:195-206. [Crossref] [PubMed]
- Briggs C, Kimber S, Green L. Where are we at with point- of- care testing in haematology? Br J Haematol 2012;158:679-90. [Crossref] [PubMed]
- Chen J, Gorman M, O’Reilly B, et al. Analytical evaluation of the epoc® point-of-care blood analysis system in cardiopulmonary bypass patients. Clin Biochem 2016;49:708-12. [Crossref] [PubMed]
- Benes J, Zatloukal J, Kletecka J. Viscoelastic Methods of Blood Clotting Assessment-A Multidisciplinary Review. Front Med (Lausanne) 2015;2:62. [PubMed]
- Chee YL, Greaves M. Role of coagulation testing in predicting bleeding risk. Hematol J Off J Eur Haematol Assoc 2003;4:373-8. [PubMed]
- Hartert H. Blutgerinnungsstudien mit der Thrombelastographie, einem neuen Untersuchungsverfahren. Klin Wochenschr 1948;26:577-83. [Crossref] [PubMed]
- Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg 1985;64:888-96. [Crossref] [PubMed]
- Lang T, Bauters A, Braun SL, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis 2005;16:301-10. [Crossref] [PubMed]
- Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol 2005;27:81-90. [Crossref] [PubMed]
- Wikkelsø A, Wetterslev J, Møller AM, et al. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. In: Afshari A. editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd, 2016:CD007871.
- Williams B, McNeil J, Crabbe A, et al. Practical Use of Thromboelastometry in the Management of Perioperative Coagulopathy and Bleeding. Transfus Med Rev 2017;31:11-25. [Crossref] [PubMed]
- Theusinger OM, Levy JH. Point of Care Devices for Assessing Bleeding and Coagulation in the Trauma Patient. Anesthesiol Clin 2013;31:55-65. [Crossref] [PubMed]
- Ogawa S, Szlam F, Chen EP, et al. A comparative evaluation of rotation thromboelastometry and standard coagulation tests in hemodilution-induced coagulation changes after cardiac surgery. Transfusion 2012;52:14-22. [Crossref] [PubMed]
- Nakayama Y, Nakajima Y, Tanaka KA, et al. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br J Anaesth 2015;114:91-102. [Crossref] [PubMed]
- Gorlinger K, Dirkmann D, Solomon C, et al. Fast interpretation of thromboelastometry in non-cardiac surgery: reliability in patients with hypo-, normo-, and hypercoagulability. Br J Anaesth 2013;110:222-30. [Crossref] [PubMed]
- Olde Engberink RH, Kuiper GJ, Wetzels RJ, et al. Rapid and correct prediction of thrombocytopenia and hypofibrinogenemia with rotational thromboelastometry in cardiac surgery. J Cardiothorac Vasc Anesth 2014;28:210-6. [Crossref] [PubMed]
- Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care 2016;20:100.
- Leemann H, Lustenberger T, Talving P, et al. The role of rotation thromboelastometry in early prediction of massive transfusion. J Trauma 2010;69:1403-8-9.
- Schöchl H, Cotton B, Inaba K, et al. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care 2011;15:R265. [Crossref] [PubMed]
- Pezold M, Moore EE, Wohlauer M, et al. Viscoelastic clot strength predicts coagulation-related mortality within 15 minutes. Surgery 2012;151:48-54. [Crossref] [PubMed]
- Davenport R, Manson J, De’Ath H, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med 2011;39:2652-8. [Crossref] [PubMed]
- Cotton BA, Faz G, Hatch QM, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma 2011;71:407-14; discussion 414-7. [Crossref] [PubMed]
- Chapman MP, Moore EE, Moore HB, et al. The “Death Diamond.” J Trauma Acute Care Surg 2015;79:925-9. [Crossref] [PubMed]
- Hunt H, Stanworth S, Curry N, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding. In: Hunt H. editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd, 2015:CD010438.
- Da Luz LT, Nascimento B, Shankarakutty AK, et al. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit Care 2014;18:518. [Crossref] [PubMed]
- Whiting P, Al M, Westwood M, et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19:1-228. v-vi. [Crossref] [PubMed]
- Hans GA, Besser MW. The place of viscoelastic testing in clinical practice. Br J Haematol 2016;173:37-48. [Crossref] [PubMed]
- De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology 2016;63:566-73. [Crossref] [PubMed]
- Müller MC, Meijers JC, Vroom MB, et al. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care 2014;18:R30. [Crossref] [PubMed]
- Brenner T, Schmidt K, Delang M, et al. Viscoelastic and aggregometric point-of-care testing in patients with septic shock - cross-links between inflammation and haemostasis. Acta Anaesthesiol Scand 2012;56:1277-90. [Crossref] [PubMed]
- Adamzik M, Langemeier T, Frey UH, et al. Comparison of thrombelastometry with simplified acute physiology score II and sequential organ failure assessment scores for the prediction of 30-day survival: a cohort study. Shock 2011;35:339-42. [Crossref] [PubMed]
- Ostrowski SR, Windeløv NA, Ibsen M, et al. Consecutive thrombelastography clot strength profiles in patients with severe sepsis and their association with 28-day mortality: A prospective study. J Crit Care 2013;28:317.e1-317.e11. [Crossref] [PubMed]
- Karlsson O, Jeppsson A, Hellgren M. Major obstetric haemorrhage: monitoring with thromboelastography, laboratory analyses or both? Int J Obstet Anesth 2014;23:10-7. [Crossref] [PubMed]
- Collins PW, Lilley G, Bruynseels D, et al. Fibrin-based clot formation as an early and rapid biomarker for progression of postpartum hemorrhage: a prospective study. Blood 2014;124:1727-36. [Crossref] [PubMed]
- Allard S, Green L, Hunt BJ. How we manage the haematological aspects of major obstetric haemorrhage. Br J Haematol 2014;164:177-88. [Crossref] [PubMed]
- Rafiq S, Johansson PI, Ostrowski SR, et al. Hypercoagulability in patients undergoing coronary artery bypass grafting: prevalence, patient characteristics and postoperative outcome. Eur J Cardiothorac Surg 2012;41:550-5. [Crossref] [PubMed]
- Hincker A, Feit J, Sladen RN, et al. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care 2014;18:549. [Crossref] [PubMed]
- Davies NA, Harrison NK, Sabra A, et al. Application of ROTEM to assess hypercoagulability in patients with lung cancer. Thromb Res 2015;135:1075-80. [Crossref] [PubMed]
- Van Haren RM, Valle EJ, Thorson CM, et al. Hypercoagulability and other risk factors in trauma intensive care unit patients with venous thromboembolism. J Trauma Acute Care Surg 2014;76:443-9. [Crossref] [PubMed]
- Levy JH, Faraoni D, Spring JL, et al. Managing New Oral Anticoagulants in the Perioperative and Intensive Care Unit Setting. Anesthesiology 2013;118:1466-74. [Crossref] [PubMed]
- Crapelli GB, Bianchi P, Isgrò G, et al. A Case of Fatal Bleeding Following Emergency Surgery on an Ascending Aorta Intramural Hematoma in a Patient Taking Dabigatran. J Cardiothorac Vasc Anesth 2016;30:1027-31. [Crossref] [PubMed]
- Herrmann R, Thom J, Wood A, et al. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Thromb Haemost 2014;111:989-95. [Crossref] [PubMed]
- Grottke O, van Ryn J, Spronk HM, et al. Prothrombin complex concentrates and a specific antidote to dabigatran are effective ex-vivo in reversing the effects of dabigatran in an anticoagulation/liver trauma experimental model. Crit Care 2014;18:R27. [Crossref] [PubMed]
- Eller T, Busse J, Dittrich M, et al. Dabigatran, rivaroxaban, apixaban, argatroban and fondaparinux and their effects on coagulation POC and platelet function tests. Clin Chem Lab Med 2014;52:835-44. [Crossref] [PubMed]
- Tarandovskiy ID, Balandina AN, Kopylov KG, et al. Investigation of the phenotype heterogeneity in severe hemophilia A using thromboelastography, thrombin generation, and thrombodynamics. Thromb Res 2013;131:e274-80. [Crossref] [PubMed]
- Sørensen B, Ingerslev J. Tailoring haemostatic treatment to patient requirements - an update on monitoring haemostatic response using thrombelastography. Haemophilia 2005;11 Suppl 1:1-6. [Crossref] [PubMed]
- Tran HT, Sørensen B, Bjørnsen S, et al. Monitoring bypassing agent therapy - a prospective crossover study comparing thromboelastometry and thrombin generation assay. Haemophilia 2015;21:275-83. [Crossref] [PubMed]
- Görlinger K, Bergmann L, Dirkmann D. Coagulation management in patients undergoing mechanical circulatory support. Best Pract Res Clin Anaesthesiol 2012;26:179-98. [Crossref] [PubMed]
- Nair P, Hoechter DJ, Buscher H, et al. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J Cardiothorac Vasc Anesth 2015;29:288-96. [Crossref] [PubMed]
- Boyle AJ, Russell SD, Teuteberg JJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant 2009;28:881-7. [Crossref] [PubMed]
- Schmid C, Hammel D, Deng MC, et al. Ambulatory care of patients with left ventricular assist devices. Circulation 1999;100:II224-8. [Crossref] [PubMed]
- Fries D, Innerhofer P, Streif W, et al. Coagulation monitoring and management of anticoagulation during cardiac assist device support. Ann Thorac Surg 2003;76:1593-7. [Crossref] [PubMed]
- Niemi TT, Kukkonen SI, Hämmäinen PT, et al. Whole blood hypercoagulability despite anticoagulation during mechanical cardiac assist. Perfusion 2008;23:107-10. [Crossref] [PubMed]
- Majeed F, Kop WJ, Poston RS, et al. Prospective, observational study of antiplatelet and coagulation biomarkers as predictors of thromboembolic events after implantation of ventricular assist devices. Nat Clin Pract Cardiovasc Med 2009;6:147-57. [Crossref] [PubMed]