Debranching aortic surgery
Introduction
Complex aortic aneurysms, including arch and thoracoabdominal aortic aneurysms (TAAAs), depending on the degree of involvement of the supra-aortic trunks (SAT) or intestinal and renal vessels, pose a tremendous challenging repair for cardiac and vascular surgeons, so different alternatives using endovascular approaches have been developed. Hybrid procedures using debranching techniques can be considered one of them. These approaches employed for the treatment of complex aneurysms affecting the aortic arch and thoracoabdominal aorta are herein separately analyzed with the objective to review their current role in the management of both pathologies.
Arch debranching
Open surgery continues to be the current “gold standard” for treatment of arch pathology (mainly aneurysms and dissections), with which the rest of available techniques have to be compared to. In most of the cases, it requires extracorporeal circulation (ECC), with or without circulatory arrest and revascularization of the SAT, and the need of performing an “elephant trunk” or a “frozen elephant trunk” when the descending aorta is also involved. Thanks to advances occurred, especially in cerebral protection techniques (antegrade cerebral perfusion), these procedures have achieved good results in high volume centers with extensive experience. However, overall results in the daily practice seem to be worse; even more, if it is taken into account that high-risk patients are unable to overcome this type of surgery, between 20–40% of all patients are excluded for repair due to their age and comorbidities, and half of them will die due to aneurysm rupture (1).
The aortic arch continues to be the proximal limit for endovascular treatment; however, recently different lesser invasive techniques have emerged, such as hybrid procedures (including debranching techniques) or even a complete endovascular repair (endografts with scallops, fenestrations, branches and parallel techniques). At the moment, endovascular treatment in this territory is only justified in high risk patients, however technological advances and increased experience have led to a growing expansion of endovascular procedures in the aortic arch for standard risk patients, which arises specific issues that will be commented on here.
General considerations for aortic arch debranching
There is consolidated experience reporting good results with endovascular treatment of descending thoracic aorta when proximal sealing is performed in Ishimaru’s zones 3 and 4 (2); which have encouraged surgeons to perform surgical procedures in order to create a safe landing area for endografts in zones 0 and 1. Nowadays, this kind of hybrid procedures, with total or partial arch debranching are well accepted in high-risk patients and there are several options to debranch the SAT, with grafts that can be routed through an anatomical or extra-anatomical path and they can be either intra or extra-thoracic. Generally, the election of the technique will depend on the characteristics of the diseased arch with regard to Ishimaru’s classification:
- For sealing in zone 0: SAT are revascularized by means of a sternotomy with complete arch debranching, by a chimney for the brachiocephalic trunk plus extra-anatomical partial debranching, or with branched or fenestrated endoprosthesis.
- When sealing in zone 1: a right to left common carotid artery (CCA) bypass with retro-esophageal or subcutaneous tunneling would be adequate, which can be associated with revascularization (bypass or transposition) of the left subclavian artery (LSA), unless the use of chimneys or specially designed endografts would be preferred.
- When sealing in zone 2: an extra-anatomical left CCA-LSA bypass or a transposition of the LSA into the left CCA prior to the placement of an endoprosthesis would be sufficient for a safe sealing.
Those patients that would require sealing in zone 0 are the most challenging cases. If a procedure by median sternotomy and bypass to all the SAT is decided, several alternatives are possible, depending on whether or not the creation of a new proximal sealing zone would be required, and the degree of involvement of the descending aorta. In spite of the fact that there are various classifications, they can basically be divided into three groups (3).
- Type I hybrid debranching: a dacron graft is directly sutured onto the ascending aorta using a partial clamping without the need for ECC, from which all the branches to SAT arise, with or without revascularization of the LSA.
- Type II hybrid debranching: in those cases with a diseased ascending aorta, it is replaced under ECC by a prosthetic graft, from which branches to SAT arise.
- Type III arch hybrid procedure: when concomitant descending aorta involvement exists, a classical open arch repair can be performed using an elephant trunk technique, or frozen elephant trunk with dedicated hybrid grafts.
These procedures may be performed during one or two stages. The risk of complications seems to be lower, if they are performed in two steps (4,5). Moreover, in cases where the aneurysm affects the descending aorta, the recovery time between both surgeries would be shorter, and then, minimizing the mortality risk from rupture meanwhile.
Once the debranching operation has been performed, several technical issues must be considered when deploying the endoprosthesis. When it is needed to seal in zone 0 and the intervention is performed on a retrograde fashion during two stages, it is recommended to place a high support stiff guide wire inside the ventricle. However, control of this guide wire must never be lost given the potential risk of myocardial perforation with devastating consequences. There is also risk of aortic valve impairment, due to the fact that it is frequently necessary to cross the aortic valve with the “nose” of the device. So, avoiding pitfalls during navigation and placement of the device in this area is an extremely important consideration to take into account. With the objective to avoid damage to the aortic valve during a single step procedure, a dacron graft can be sutured close to the aortic root through which guide wires and the endograft can be introduced, obtaining good stability of the system. This measure can be especially useful in the case of previously prosthetic aortic valve replacement. If a partial debranching using chimneys is decided, apparently this procedure would offer several advantages: it permits sealing in zone 0 without sternotomy, it is an easier procedure than fenestrated or branched endografts and it uses conventional devices, allowing its application in urgent cases. However, the technology for thoracic endoprosthesis was not developed to perform these kind of techniques and currently no endograft manufacturer supports its use, leaving many open questions unanswered: how do the movements of the aortic arch would affect the chimneys and the endografts? Could they cause erosion of the fabric or cause stent fracture? How much should the endograft be oversized? Ideal type of stents? etc.
The deployment of the endograft must be extremely precise, and for this purpose a careful planning, including a meticulous study of the angulations to correctly adjust the endograft in the aortic arch with respect to the origin of the debranching or the SAT, is required. When performing a debranching procedure through a sternotomy, it is advisable to use a lead marker of a gauze in order to easily identify the takeoff of the debranching, so that the deployment of the endograft can be performed as closest as possible to it. It is also recommended to reduce the blood pressure at the moment of the deployment. In zone 0, usually, a rapid pacing is preferred while for zones 1 and 2 controlled hypotension is performed before the delivery of the device.
Whenever the surgical procedure is elective, revascularization of the LSA should be performed as well as the use of selective cerebrospinal fluid (CSF) drainage, which will be used depending on the length of the descending aorta that will be covered, on whether there is no adequate revascularization of the LSA and if the patient has undergone prior abdominal aortic surgery (lumbar arteries occlusion) or if the hypogastric arteries are occluded.
With this general view of the surgical technique in mind, it would appear that debranching procedures are clearly advantageous compared not only with conventional open surgery, but, also with pure endovascular techniques, due to the fact that they use conventional endografts, which aside from being cheaper, they avoid delay in surgery by not having to wait for custom made devices and are ready to be used in a wide range of anatomies. However, the need for lateral clamping of the ascending aorta causes a potential injury to the artery which, mainly if it is associated to the stress forces generated by the endoprosthesis when it lands upon this aortic zone, can produce a retrograde type A dissection (3-5) in up to 6% of the cases, of which 42% are lethal (5). In addition, over time, these lesions may cause aneurysmal degeneration, eventually resulting in a type I endoleak. This complication can be avoided if a hybrid type II debranching procedure with prosthetic replacement of the ascending aorta is performed (6).
Therefore, these procedures must be considered a major surgical operation with potential serious complications. In addition, not all patients are adequate candidates for this type of treatment because certain conditions are required to be able to perform an endovascular or hybrid treatment. General indications accepted for hybrid surgery include elderly patients with important comorbidities and favorable anatomical factors (ascending aorta above 42 mm are not suitable for sealing as currently standard devices have a maximum diameter of 46 mm, even an ascending aorta wider than 37 mm may be discarded given the higher rate of retrograde dissections and complications from the lateral clamping in this setting) (6). The maximum diameter of the ascending aorta is the most important exclusion criteria. Given that isolated aortic arch aneurysms are scarce, it is not easy to fulfill those previously mentioned requirements and then, frequently a type II debranching procedure must be performed so, in the elderly and in those patients with important risk factors, many of the advantages of the hybrid treatment could be lost, despite cerebral ischemia time is shorter than in conventional surgery. In a series by De Rango et al. (7), only 17% of patients in the open surgery group met the criteria for endovascular treatment without replacement of the ascending aorta, while in another recent study from Sweden (8), only 7% of them were considered to be adequate candidates for treatment without making a safe landing zone by means of a dacron graft in the ascending aorta.
Current results
Present data regarding the results of hybrid procedure in the aortic arch are not easy to interpret, comprising heterogeneous cases with short series or retrospective studies showing disparate results. The first difficulty arises when deciding to what the results should be compared to. As we previously mentioned, technical improvements in cerebral perfusion and protection brought an important reduction in morbidity-mortality rates for open surgery performed in centers of excellence. Settepani et al. (1) showed a 5% mortality rate in a systematic literature review covering the last ten years. Nevertheless, data from the National Impatient Sample (9,10) and the Medicare Provider Analysis and Review (11,12) reflect very different results, with worldwide mortality rates ranging from 15% to 20%.
Even more, studies comparing both techniques show short retrospective series and very heterogeneous groups, regarding patients’ age and surgical risks. Usually, patients in the endovascular group are older and with more comorbidities. Age is an important predictor of operative mortality for these pathologies, as reported by Milewski et al. (13), with a mortality rate four times lower in patients younger than 75 years old (9%) than in those above that age (36%).
There are not many studies available comparing the traditional arch repair technique with modern hybrid techniques. Moreover, in most of the latter, total and partial debranching techniques are mixed together, as well as the different sealing zones. De Rango et al. (7) reported 100 consecutive patients treated at the same center during seven consecutive years, including 29 cases in the open surgery group and 71 in the endovascular group (7 patients with chimneys and 64 with total or partial debranching). Even though, both groups were not equivalent with regard to surgical risks (the open surgery group was younger and with lower preoperative comorbidities), no differences were found regarding operative mortality and 30-day stroke risk. Similar results were reported by Milewski et al. (13), including a debranching procedure in 45 cases and 27 patients in the traditional open repair group. On the other hand, more controversy was added by Benedetto et al. (14), concluding in a meta-analysis that the hybrid arch treatment does not reduce the immediate mortality rate and increases the risk of stroke.
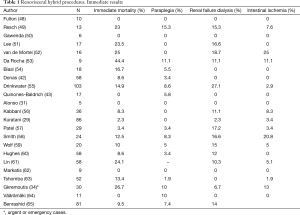
With respect to surgical complications for hybrid procedures, neurological problems, compromising the brain, spinal cord or both, deserve a special mention. Stroke will be more frequent whenever manipulation of the SAT and the aortic arch are performed, and also depending on the presence of previous lesions at that level. The reported incidence of this complication is very variable, reaching percentages as high as 12% (5,15) and it increases in those cases where sealing is attempted in zones 0 or 1. On the other hand, paraplegia rate seems to be lower than for classical open repair and it usually does not exceed 3% (15,16). Revascularization of the LSA, combined with CSF drainage and preservation of intercostal circulation by using endograft devices as short as possible, apart from avoiding anemia, hypotension and vasoconstrictor medications, are a useful tool to reduce the rate of this devastating complication.
The medium and long-term results for these techniques are not well established yet. De Rango et al. (7) did not find any difference between the endovascular and open surgery groups, although the report by Sood et al. (17) showed a higher rate of re-interventions and aortic rupture in the former group. Obviously, these problems could be related to an inadequate sealing by the endoprosthesis, leading to an incomplete exclusion of the aneurysmal sac. However, the incidence of this complication is much lower in other series; like the reported one by Czerny et al. (18), consisting of a transcontinental registry with a mean follow up of 25 months (from 8 to 41 months) and being described just one late type Ia endoleak. The reported 5-year survival rate was 96%. In the report by Vallejo et al. (19), including 38 aortic arch hybrid procedures during a mean follow up of 28.1 months (range, 1–84 months), four type I endoleaks and two type II were described. On the other hand, Andersen et al. (20), after a mean follow-up of 28 months, found that 11 out of 87 patients (13%) required a redo-procedure because of endoleaks. Bavaria et al. (21) noticed just one case of re-intervention, with no type I or III endoleaks, in patients treated by hybrid approaches for aortic arch pathology, after a mean follow up of 30 months. It seems clear that the presence of a type I endoleak is more frequent in cases sealing in zones 1 and 2, compared with those where it was performed in zone 0 (7,15,18). If sealing is extended to Ishimaru’s zone 0 or 1, usually, the endograft is much more stable than when it only reaches zones 2 or 3, directly on the arch curvature and leading to a higher number of complications. Nevertheless, it is necessary to remind that sealing in more proximal zones of the arch, even when achieving better stability for the endoprosthesis, as a counterpart, it also increases the risk of intra-operative complications, in particular neurological events.
Another possible and serious complication is the development of a retrograde type A dissection; more frequent in those patients that sealing takes place in zone 0. There are several predisposing factors for a retrograde dissection, ranging from aortic wall lesions attributed to the endovascular device to the damage originated by the use of guide wires and catheters, or by the lateral clamping of the ascending aorta for bypass suture, as it has already been mentioned. In the series by Andersen et al. (20), an aortic dissection appeared in 6.3% of the patients, but increased up to 11% when excluding those with previously performed replacement of the ascending aorta. Therefore, careful attention about this fact must be required during follow up.
Finally, there is not too much information available about the long-term evolution of the aortic arch aneurysm after an endovascular or hybrid treatment. The absence of aneurysm growth (understood as an increase in the aortic diameter over 5 mm), was achieved in 96.7% of the cases during a 5-year follow up (22).
Renovisceral debranching
Following the outstanding improvements in the technique by Crawford in the eighties, open surgery still remains the gold standard procedure for repair of TAAAs (23). Later, and over time, continuous advances have been introduced in order to reduce complications due to intestinal ischemia, kidneys, lower limbs and spinal cord ischemia. In spite of this, conventional surgery for TAAAs is still associated with high morbidity and mortality rates, even in centers with a large experience with thoracoabdominal repair. And when population studies are considered, data are even worse, with immediate mortality rates of 20% at the first month and up to 30% during the first year (10); taking into account that other devastating complications (renal failure, paraplegia) can happen. Coselli’s group (24) represents the excellence with more than two thousand patients operated with rates of spinal cord ischemia ranging from 2.7% to 13.2%, depending on the type of the aneurysm; renal failure needing dialysis from 4.6% to 5.6% and 30-day mortality from 5% to 19%. For these reasons, and being aware of that endovascular treatment for thoracic and abdominal aneurysms has significantly decreased the immediate mortality, hybrid procedures (renovisceral debranching plus endovascular exclusion) have emerged as an alternative technique for the treatment of TAAAs in the endovascular era.
Initially hybrid procedures were performed in high-risk patients unfit for open repair due to several theoretical advantages: (I) minimal hemodynamic repercussion because they avoid an aortic cross-clamping at a high level; (II) they preclude a long and simultaneous renovisceral ischemic insult; (III) a thoracoabdominal approach with left lung collapse is not needed, meaning a lesser surgical aggression; and (IV) a lower incidence of paraplegia. Compared with pure endovascular techniques, it has already been mentioned that debranching procedures are easier to perform, they use conventional devices with no delay for treatment of urgent cases and they allow to be applied to a wide variety of anatomies.
Current status of renovisceral debranching procedures for thoracoabdominal aortic aneurysms
Renovisceral hybrid procedures for TAAAs must mainly respond to the criticism regarding.
Morbidity-mortality
One of the first larger series about renovisceral debranching was published in 2006 from the St Mary’s Hospital, including 29 patients (10% of them urgent cases) with extensive TAAAs (type IV were excluded), reporting a 13% immediate mortality rate and with no paraplegia. However, authors revealed they obtained worse results with their later experience (25).
Patel et al. (26) showed severe criticism of the hybrid treatment for TAAAs. They compared a group of high-risk patients for conventional surgery in whom a hybrid procedure was performed with another group of patients with conventional open repair, and they found that the combined rate of mortality-paraplegia was double in the hybrid group (21.7% hybrid vs. 11.7% conventional). The interpretation of this study must be performed cautiously given the important bias that exists when comparing patients with more comorbidities and more complex and extensive TAAAs in the hybrid group (61% type I or type II and it did not include any type IV), with another group of completely different patients in which type IV TAAAs represented 34% of the cases, whilst type I and II aneurysms only accounted for 28% of them.
Likewise, the results of a meta-analysis by Moulakakis et al. (27) including a total of 528 patients seem to be little enthusiastic (tough, it must be taken into account that 11% of them were urgent or emergency procedures): in 3 cases the intervention was not completed due to intraoperative instability, 12 deaths were related with the procedure of visceral revascularization, 6 other patients died due to aortic rupture while waiting for endovascular exclusion and 4 cases rejected the second stage for completion of the procedure. The 30-day cumulative mortality rate was 14% (68 patients) and spinal cord ischemia was present in 7% of the cases, with a 4.5% rate of irreversible paraplegia. Also, the North American Complex Abdominal Aortic Debranching Registry (NACAAD) showed similar figures of mortality (16%) with a 14% rate of paraplegia (28).
On the other hand, there are several individual series where excellent results were achieved; reporting immediate mortality rates ranging from 2% to 4% (29,30). In 2010, we reported our preliminary experience with renovisceral hybrid procedures for extensive TAAAs with neither early mortality nor paraplegia (31). Hughes et al. (32), who performed these procedures in 47 high surgical risk patients, reported 30-day mortality and spinal cord ischemia rates of 8.5% and 4.3%, respectively. There were no deaths, paraplegia or paresis in a subgroup of 14 patients in which the intervention was carried out in two stages.
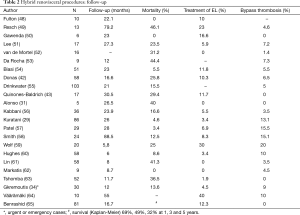
A recent meta-analysis including 660 patients (33) suggested that staged procedures are advantageous, although without statistical significance, due to the fact that many studies do not contemplate this difference in their results and there is an insufficient sample size. Some authors advocate for a one stage strategy and base their position on: the risk of aortic rupture during the delay between interventions, the possible refusal by the patient to undertake the endovascular stage, the possibility of using a trans-abdominal approach in order to deploy the endoprosthesis in those cases with iliac compromise, and the risk of damaging the retrograde bypasses during a transfemoral access. Nevertheless, a two-stage procedure allows for a shorter duration of the intervention with lower renal impairment (avoiding the injection of contrast immediately after renal ischemia), it also improves haemodynamic stabilization of the patient, reducing the risk of spinal ischemia by avoiding hypotension, and permits a prompt evaluation for spinal cord ischemia after aortic coverage. Apart from all these reasons in the eventual case of a poor outcome, the expenses associated with the endoprosthesis and the endovascular procedure can be avoided.
In the aforementioned meta-analysis, operative mortality was reported as 12.6% (0–44%), the incidence of paraplegia was 3.4% (0–15.3%) and the rates of permanent renal failure, mesenteric ischemia and severe cardiopulmonary complications were 10.4% (0–27.1%), 4.6% (0–20.8%) and 7.8% (0–17.6%), respectively.
Furthermore, a recent report including 30 urgent or emergency patients, considers hybrid repair procedures a valid treatment alternative for complex thoracoabdominal pathology in these settings, showing a 30-day mortality rate of 36.8% in the emergency patients and 9% in the urgent group (34).
Although several safety studies on thoracic endoprosthesis reported a lower incidence of spinal cord ischemia and paraplegia compared with open surgery (6% vs. 10%) (35-37); endovascular repair of thoracic aorta and TAAAs is still associated with a significant risk of paraplegia, reaching rates of 13% in a systematic review by Wong et al. (38). Hybrid treatment offers better results with regard to the risks of permanent paraplegia and paresis than the majority of large series on open repair [only comparable to the best series reported by Coselli et al. (24) with 3.8%], without forgetting that cases undergoing hybrid procedures are often classified as TAAAs type I or II, a group of patients with paraplegia rates as high as 16% with conventional surgery. The lower incidence of spinal cord ischemia for hybrid procedures can be explained in base to its multifactorial etiology (39), in which perioperative hypotension and prolonged ischemia of the lower half of the body are crucial factors. Both are potentially avoidable with renovisceral debranching. Prompt recovery of pelvic and lower limbs perfusion as a mean to reduce the incidence of spinal cord ischemia has been underestimated, as shown by Maurel et al. (40) on their series of endovascular repair of type I, II and III TAAAs (excluding type IV), where the rate of spinal cord ischemia was reduced from 25% to 2% when this measure was adopted, together with CSF drainage, an aggressive protocol for transfusion and maintaining a mean arterial blood pressure above 85 mmHg.
In any case, considering the lack of homogeneity in the available data, it is not possible to confirm that the incidence of spinal ischemia is lower for hybrid procedures than for conventional surgery with full certainty.
Graft patency
Patency of renal and visceral grafts is another key element in these procedures. There is limited data considering current results on conventional surgery for renal or mesenteric arteries, because nowadays, the great majority of these vessels are revascularized by means of endovascular techniques. Generally speaking, the long-term patency of bypasses to renal and other visceral arteries is placed around 90% (41).
On the other hand, the available literature on hybrid procedures, although inconsistent in terms of follow-up, provides primary patency rates over 95% (27,42,43). A meta-analysis performed by Canaud et al. (33) showed excellent patency rates (94.7%) for these vascular reconstructions during a mean follow-up of 26.2 months (6–88.5 months). In the same manner, Shahverdyan et al. (44) reported good primary patency rates after 5 years on 46 patients, although it was lower for the right renal artery (global patency 86.1%±3.1%, hepatic patency 100%, superior mesenteric artery 88.8%±4.8%, left renal artery 87.2%±6% and right renal artery 69.6%±8.8%). Usually, the right renal artery anastomosis is technically more complex, and therefore demanding special care when it is performed, and even the use of alternative techniques can be contemplated. In 2008, Lachat et al. (45) described the VORTEC technique (Viabahn Open Rebranching TEChnique) for the revascularization of renal arteries, allowing for a fast and simple procedure with better haemodynamic results at experimental level, and which has significantly improved with the introduction of the new Gore Hybrid Vascular Grafts (W.L. Gore and Associates, Flagtaff, Ariz). It should also be mentioned that in order to facilitate the endovascular technique during the repair of complex and extensive TAAAs, occasionally it may be possible to perform a VORTEC technique in the renal arteries together with complementary endovascular repair including branched or fenestrated endografts for the rest of the visceral trunks. As drawbacks, the VORTEC technique significantly increases the costs of the procedure when four-vessel revascularization is needed, and the fact that, the hybrid vascular grafts are long prosthesis with a relatively small diameter (6 mm) in the stentless segment, with the potential increased risk of stenosis in case of minimal hyperplasia. At present, there is no clinical evidence regarding the superiority of patency rates achieved with this technique. A recent report showed no stent graft fractures or secondary dislocation and an 84.7%±5.2% cumulative patency rate at 89 months after VORTEC (46).
Endoleaks
Endoleaks can reduce the effectiveness of the endovascular treatment and they are another critical point to take into account when renovisceral debranching procedures are going to be considered. In the meta-analysis performed by Moulakakis et al. (27); after a mean follow-up of 34.2 months, in 502 patients who underwent the procedure, 21% of them presented endoleaks (59% type II, 29% type I and 11% type III), requiring re-intervention in one fourth of the cases. Nevertheless, endoleaks pose a common problem to all modalities of endovascular treatment and, for thoracic aortic procedures, they have been reported in the literature with a variable incidence, ranging from 5% to 26% at 30 days (mean 10%) and from 4% and 28% after one year (mean 10%) (47). However, the potential risk of endoleaks for hybrid debranching procedures should be expected to be lower than for other endovascular modalities of treatment for TAAAs, taking into account that debranching techniques do not require the deployment of renovisceral covered-stents to maintain patency of these critical branches, which at the long-term are not an infrequent cause of endoleaks. In order to minimize the incidence of type I and III endoleaks (those considered as failure) it is necessary to obtain a secure sealing zone, both proximally and distally, as well as a sufficient overlapping between devices.
Tables 1,2 show early and late results for renovisceral debranching procedures, making it evident that these techniques must be considered as complex surgery with significant mortality and morbidity. In any case, these results are probably better than those that would have been obtained with conventional surgery performed in the same patients, although there is no sufficient scientific evidence. In a recent report comparing 84 conventional open and 81 hybrid repairs, despite the greater comorbidity burden in the hybrid group, with a significantly higher rate of preoperative chronic renal failure (a well-known independent predictor of mortality), there were no differences in the in-hospital mortality or spinal cord ischemia rates, while patients in the conventional group presented more strokes (9.5% vs. 0%, P=0.017) and those in the hybrid group a significantly increased rate of definitive dialysis (14.8% vs. 3.6%, P=0.043) and higher reintervention rates (12.3% vs. 1.2%, P=0.004), mainly due to endoleaks. Both groups had equivalent survival rates at the first year (hybrid 69% vs. open 77%) and similar long-term aorta-specific survival, suggesting that both techniques can have complementary roles and that the coexistence of several options for treatment of TAAAs in a single centre allows for the treatment of a greater number of patients (65).
Full table
Full table
It can also be debated if performing hybrid surgery is still justified after important advances appeared in the endovascular field of TAAAs, with the development of specifically designed fenestrated and branched endografts, and the new standardized endoprosthesis (off-the shelf). Preliminary reports offer promising short-term results with these devices (66,67), although most of these series include a high percentage of type IV cases. Recently, Verhoeven et al. (68) published their experience with 166 patients, reporting an in-hospital mortality rate of 9% and a similar rate of spinal cord ischemia (9%) with 1% of permanent paraplegia. The mean follow up was 29±21 months, with two deaths in relation with the aneurysm, and need of re-intervention in 24% of the patients. Austermann et al. reported their results in 107 patients with pararenal and TAAAs; during a follow up period of over one year, they described complications in 28 of them (26%), requiring 34 re-interventions (6 branch thrombosis/stenosis, 8 visceral stent occlusions/stenosis, 8 type I and 12 type III endoleaks) (69).
Good patency rates have been reported for renal and visceral vessels after endovascular repair with fenestrated and branched aortic endoprosthesis: Verhoeven et al. (70) published a 5-year patency rate of 93%, with most occlusions occurring during the first two years and being the late renal events the main concern, responsible for a significant deterioration of renal function in up to 25% of the patients.
A recent review of the endovascular treatment for TAAAs, showed an immediate mortality rate ranging from 0 to 21%, spinal cord ischemia from 0 to 33%, and a reintervention rate between 3% and 25% (71).
It can be stated that the endovascular treatment of TAAAs, like other treatment modalities for this pathology, shows heterogeneous, although promising results, at short-term and in selected centres. Nevertheless, a significantly high re-intervention rate can be expected, further increasing as the follow-up extends, and consistent long-term results are still unavailable. Moreover these are complex procedures, very expensive, not yet widely performed and with obvious logistic difficulties. There are also anatomic limitations preventing a pure endovascular treatment, such as: a narrow aortic lumen (that could compromise branch patency), severe angulations or tortuosity, iliac occlusive disease, etc. In an attempt to palliate some of these problems, new standardized endoprosthesis (off-the-shelf) are being developed, which are expected to allow treatment from 60% to 80% of the TAAAs. Bisdas et al., using the Zenith t-Branch (Cook Incorporated, Bloomington, IN, USA), reported preliminary results quite comparable to those obtained with customized devices, although with renal stent thrombosis in 13.6% of the cases (3/22) (72).
Other less common procedures, specially the “parallel endograft” techniques (chimneys, snorkels, sandwich), have also been performed. They have been mainly used for treatment of yuxta and pararenal aneurysms and at the moment, the experience with these techniques for TAAAs is very limited. The main advantages of this type of procedures are: the use of conventional devices and the possibility of allowing catheterization of visceral branches before deploying the endoprosthesis. On the other hand, one of the main concerns for treatment of TAAAs is the imperfect or incomplete apposition between the covered stent and the endoprosthesis, causing endoleaks. In order to address this problem, several resources have been adopted: (I) an excessive oversizing of the aortic endoprosthesis, often causing infolding; or (II) extensive overlapping, increasing the risk of bypass thrombosis and unintentional occlusion of lumbar and intercostal arteries. Literature regarding these techniques in TAAAs is scarce and heterogeneous, also including yuxta and pararenal aortic aneurysms and with a small number of patients. One of the largest series was published by Lachat et al. (73) treating 77 patients, where only 20 (26%) were TAAs (6 type I). The mean follow-up period was 24 months, reporting 9 deaths from unknown causes, 26% type I/III endoleaks, 10% re-intervention rate in relation with visceral stent complications, and 4% thrombosis. At the moment, there is a general consensus that these procedures should be limited to emergency cases or unfavourable anatomies, in which other techniques are not feasible.
Conclusions
Treatment of complex aortic pathology, mainly arch and TAAAs still represents a major challenge, surrounded by controversy and with a great disparity of the results inside the different therapeutic modalities. Debranching techniques for both pathological conditions are procedures whose complexity should not be underestimated, demanding careful planning and surgical expertise in order to reduce mortality and complications. It can be expected that the development of new devices and endovascular techniques, together with increasing technical expertise by the operator, will allow treating larger numbers of patients in a pure endovascular fashion. Nevertheless, hybrid debranching procedures remain as a valuable alternative: for arch pathology, they can avoid or reduce the time of ECC or cardiac arrest which may be beneficial in high-risk patients that otherwise would be rejected for treatment and they can also allow antegrade deployment from ascending aorta, facilitating a single step procedure. When debranching arch operations are compared to pure endovascular techniques, they can be used in emergency cases with applicability in a wide range of anatomies. For treatment of TAAAs, debranching procedures are mainly useful when other less invasive endovascular options are not feasible due to anatomical limitations (narrow vascular lumen, renovisceral vessel compromised by dissection, angulations and severe tortuosity that prevent precise positioning of the endoprosthesis, limited vascular access, etc.) or when they are not available in cases where delaying the intervention is not an option.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Settepani F, Cappai A, Basciu A, et al. Outcome of open total arch replacement in modern era. J Vasc Surg 2016;63:537-45. [Crossref] [PubMed]
- Ishimaru S. Endografting of the aortic arch. J Endovasc Ther 2004;11:II62-71. [Crossref] [PubMed]
- Vallabhajosyula P, Szeto WY, Desai N, et al. Type II arch hibrid debranching, procedure. Ann Cardiothorac Surg 2013;2:378-86. [PubMed]
- Czerny M, Schmidi J, Carrel T, et al. Hybrid aortic arch repair. Ann Cardiothorac Surg 2013;2:372-7. [PubMed]
- Kent WD, Appoo JJ, Bavaria JE. Results of type II hybrid arch repair with zon 0 stent granf deployment for complex aoric arch pathology. J Thorac Cardiovasc Surg 2014;148:2951-5. [Crossref] [PubMed]
- Vallabhajosyula P, Szeto WY, Desai N, et al. Type II arch hybrid debranching procedure. Ann Cardiothorac Surg 2013;2:378-86. [PubMed]
- De Rango P, Ferrer C, Coscarella C, et al. Contemporary comparison of aortic arch repair by endovascular and open surgical reconstructions. J Vasc Surg 2015;61:339-46. [Crossref] [PubMed]
- Sonesson B, Landenhed M, Dias N, et al. Anatomic feasibility of endovascular reconstruction in aortic arch aneurysms. Vascular 2015;23:17-20. [Crossref] [PubMed]
- Sachs T, Pomposelli F, Hagberg R, et al. Open and endovascular repair of type B aortic dissection in the Nationwide Inpatient Sample. J Vasc Surg 2010;52:860-6. [Crossref] [PubMed]
- Cowan JA, Mimick JB, Henke PK, et al. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg 2003;37:1169-74. [Crossref] [PubMed]
- Patel VI, Mukhopadhyay S, Ergul E, et al. Impact of hospital volume and type on outcomes of open and endovascular repair of descending thoracic aneurysm in the United States Medicare population. J Vasc Surg 2013;58:346-54. [Crossref] [PubMed]
- Chikwe J, Cavallaro P, Itahgaki S, et al. National outcomes in acute aortic dissection: influence of surgeon and institutional volume on operative mortality. Ann Thorac Surg 2013;95:1563-9. [Crossref] [PubMed]
- Milewski RK, Szeto WY, Pochettino A, et al. Have hybrid procedures replaced open aortic arch reconstrution in high-risk patients? A comparative study of elective open arch debranching with endovascular stent graft placement and conventional elective open total and distal aortic arch reconstruction. J Thorac Cardiovasc Surg 2010;140:590-7. [Crossref] [PubMed]
- Benedetto U, Melina G, Angeloni E, et al. Current result of open total arch replacement versus hybrid thoracic endovascular aortic repair for aortic arch aneurysm: a meta-analysis of comparative studies. J Thorac Cardiovasc Surg 2013;145:305-6. [Crossref] [PubMed]
- Narita H, Komori K, Usui A, et al. Postoperative outcomes of hybrid repair in the treatment of aortic arch aneurysms. Ann Vasc Surg 2016;34:55-61. [Crossref] [PubMed]
- Cambria RP, Crawford RS, Cho JS. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of descending thoracic aorta. J Vasc Surg 2009;50:1255-64.e1. [Crossref] [PubMed]
- Sood V, Patel HJ, Williams DM, et al. Open and endovascular repair of the nontraumatic isolated aortic arch aneurysm. J Vasc Surg 2014;60:57-63. [Crossref] [PubMed]
- Czerny M, Weigang E, Sodeck G, et al. Targeting landing zone 0 by total arch rerouting and TEVAR: midterm result of a transcontinental registry. Ann Thorac Surg 2012;94:84-9. [Crossref] [PubMed]
- Vallejo N, Rodriguez-Lopez JA, Heidari P, et al. Hybrid repair of thoracic aortic lesions for zone 0 and 1 in high-risk patients. J Vasc Surg 2012;55:318-25. [Crossref] [PubMed]
- Andersen ND, Williams JB, Hanna JM, et al. Results with an algorithmic approach to hybrid repair of the aortic arch. J Vasc Surg 2013;57:655-67. [Crossref] [PubMed]
- Bavaria J, Vallabhajosyula P, Moeller P, et al. Hybrid approaches in the tratment of aortic arch aneurysm: Postoperative and midterm outcomes. J Thorac Cardiovasc Surg 2013;145:S85-90. [Crossref] [PubMed]
- De Rango P, Cao P, Ferrer C, et al. Aortic arch debranching and thoracic endovascular repair. J Vasc Surg 2014;59:107-14. [Crossref] [PubMed]
- Crawford ES, DeNatale RW. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg 1986;3:578-82. [Crossref] [PubMed]
- Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4. [Crossref] [PubMed]
- Black SA, Wolfe J, Clarck M, et al. Complex thoracoabdominal aortic aneurysms: endovascular exclusion with visceral revascularization. J Vasc Surg 2006;43:1081-9. [Crossref] [PubMed]
- Patel R, Conrad MF, Paruchuri V, et al. Thoracoabdominal aneurysm repair: Hybrid versus open repair. J Vasc Surg 2009;50:15-22. [Crossref] [PubMed]
- Moulakakis KG, Mylonas SN, Antonopoulos CN, et al. Combined open and endovascular treatment of thoracoabdominal aortic pathologies: a systematic review and meta-analysis. Ann Cardiothorac Surg 2012;1:267-76. [PubMed]
- Oderich GS, Timaran C, Farber M. Spinal cord injury after hybrid endovascular repair of thoracoabdominal aortic aneurysms in the North American Complex Abdominal Aortic Debranching (NACAAD) Registry. 2012 Vascular Annual Meeting. June 7-9, 2012, Washington DC, USA.
- Kuratani T, Kato M, Shirakawa Y, et al. Long-term results of hybrid endovascular repair for thoraco-abdominal aortic aneurysms. Eur J Cardiothorac Surg 2010;38:299-304. [Crossref] [PubMed]
- Ham SW, Chong T, Moos J, et al. Arch and visceral/renal debranching combined with endovascular repair for thoracic and thoracoabdominal aortic aneurysms. J Vasc Surg 2011;54:30-40. [Crossref] [PubMed]
- Alonso M, Llaneza JM, Camblor LA, et al. Experiencia preliminar con cirugía híbrida en el tratamiento de los aneurismas toracoabdominales. Angiología 2010;62:45-50. [Crossref]
- Hughes GC, Barfield ME, Shah AA, et al. Staged total abdominal debranching and thoracic endovascular aortic repair for thoracoabdominal aneurysm. J Vasc Surg 2012;56:621-9. [Crossref] [PubMed]
- Canaud L, Karthikesalingam A, Jackson D, et al. Clinical outcomes of single versus staged hybrid repair for thoracoabdominal aortic aneurysm. J Vasc Surg 2013;58:1192-200. [Crossref] [PubMed]
- Gkremoutis A, Schmandra T, Meyn M, et al. Hybrid approach to emergent and urgent treatment of complex thoracoabdominal aortic pathology. Eur J Vasc Endovasc Surg 2014;48:407-13. [Crossref] [PubMed]
- Makaroun MS, Dillavou ED, Kee ST, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 2005;41:1-9. [Crossref] [PubMed]
- Matsumura JS, Cambria RP, Dake MD, et al. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg 2008;47:247-57. [Crossref] [PubMed]
- Fairman RM, Criado F, Farber M, et al. Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System: the VALOR trial. J Vasc Surg 2008;48:546-54. [Crossref] [PubMed]
- Wong CS, Healy D, Canning C, et al. A systematic review of spinal cord injury and cerebrospinal fluid drainage after thoracic aortic endogrfating. J Vasc Surg 2012;56:1438-47. [Crossref] [PubMed]
- Safi HJ, Miller CC 3rd, Carr C, et al. Importance of intercostal artery reattachment during thorcoabdominal aortic aneurysm repair. J Vasc Surg 1998;27:58-66. [Crossref] [PubMed]
- Maurel B, Delclaux N, Sobocinski J, et al. The impact of early pelvic and lower limb reperfusion and attentive peri-operative management on the incidence of spinal cord ischemia during thoracoabdominal aortic aneurysm endovascular repair. Eur J Vasc Endovasc Surg 2015;49:248-54. [Crossref] [PubMed]
- Hansen KJ, Wong JM. Aortorenal bypass for renovascular hypertension in adults. In: Ernst CB, Stanley JC. editors. Current therapy in vascular surgery. 4th ed. St Louis, MO: Mosby, 2001:735.
- Donas KP, Lachat M, Rancic Z, et al. Early and midterm outcome of a novel technique to simplify the hybrid procedures in the treatment of thoracoabdominal and pararenal. J Vasc Surg 2009;50:1280-4. [Crossref] [PubMed]
- Quinones-Baldrich W, Jimenez JC, DeRubertis B, et al. Combined endovascular and surgical approach (CESA) to thoracoabdominal aortic pathology: a 10-year experience. J Vasc Surg 2009;49:1125-34. [Crossref] [PubMed]
- Shahverdyan R, Gawenda M, Brunkwall J. Five-year patency rates of renal and visceral bypasses after abdominal debranching for thoraco-abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2013;45:648-56. [Crossref] [PubMed]
- Lachat M, Mayer D, Criado FJ, et al. New technique to facilitate renal revascularization with use of telescoping self-spanding stent grafts: VORTEC. Vascular 2008;16:69-72. [Crossref] [PubMed]
- Winklehner A, Nguyen-Kim TD, Pfammatter T, et al. Graft Patency in long-term survivors after renovisceral debranching with VORTEC. Cardiovasc Intervent Radiol 2015;38:606-12. [Crossref] [PubMed]
- Ricotta JJ. Endoleak management and postoperative surveillance following endovascular repair of thoracic aortic aneurysms. J Vasc Surg 2010;52:91S-99S. [Crossref] [PubMed]
- Fulton JJ, Farber MA, Marston WA, et al. Endovascular stent-graft repair of pararenal and type IV thoracoabdominal aortic aneurysms with adjunctive visceral reconstruction. J Vasc Surg 2005;41:191-8. [Crossref] [PubMed]
- Resch TA, Greenberg RK, Lyden SP, et al. Combined staged procedures for the treatment of thoracoabdominal aneurysms. J Endovasc Ther 2006;13:481-9. [Crossref] [PubMed]
- Gawenda M, Aleksic M, Heckenkamp J, et al. Hybrid-procedures for the treatment of thoracoabdominal aortic aneurysms and dissections. Eur J Vasc Endovasc Surg 2007;33:71-7. [Crossref] [PubMed]
- Lee WA, Brown MP, Martin TD, et al. Early results after staged hybrid repair of thoracoabdominal aortic aneurysms. J Am Coll Surg 2007;205:420-31. [Crossref] [PubMed]
- van de Mortel RH, Vahl AC, Balm R, et al. Collective experience with hybrid procedures for suprarenal and thoracoabdominal aneurysms. Vascular 2008;16:140-6. [Crossref] [PubMed]
- Da Rocha MF, Miranda S, Adriani D, et al. Hybrid procedures for complex aortic pathology: initial experience at a single center. Rev Esp Cardiol 2009;62:896-902. [PubMed]
- Biasi L, Ali T, Loosemore T, et al. Hybrid repair of complex thoracoabdominal aortic aneurysms using applied endovascular strategies combined with visceral and renal revascularization. J Thorac Cardiovasc Surg 2009;138:1331-8. [Crossref] [PubMed]
- Drinkwater SL, Böckler D, Eckstein H, et al. The visceral hybrid repair of thoracoabdominal aortic aneurysm: a collaborative approach. Eur J Vasc Endovasc Surg 2009;38:578-85. [Crossref] [PubMed]
- Kabbani LS, Criado E, Upchurch GR Jr, et al. Hybrid repair of aortic aneurysms involving the visceral and renal vessels. Ann Vasc Surg 2010;24:219-24. [Crossref] [PubMed]
- Patel HJ, Upchurch GR Jr, Eliason JL, et al. Hybrid debranching with endovascular repair for thoracoabdominal aneurysms: A comparison with open repair. Ann Thorac Surg 2010;89:1475-81. [Crossref] [PubMed]
- Smith TA, Gatens S, Andres M, et al. Hybrid repair of thoracoabdominal aortic aneurysms involving the visceral vessels: comparative analysis between number of vessels reconstructed, conduit, and gender. Ann Vasc Surg 2011;25:64-70. [Crossref] [PubMed]
- Wolf O, Eckstein HH. Combined open and endovascular treatment of thoracoabdominal aneurysms and secondary expanding aortic dissections: early and mid-term results from a single–centre series. Ann Vasc Surg 2010;24:167-77. [Crossref] [PubMed]
- Hughes GC, Andersen ND, Hanna JM, et al. Thoracoabdominal aortic aneurysm: hybrid repair outcomes. Ann Cardiothorac Surg 2012;1:311-9. [PubMed]
- Lin PH, Kougias P, Bechara CF, et al. Clinical outcome of staged versus combined treatment approach of hybrid repair of thoracoabdominal aortic aneurysm with visceral vessel debranching and aortic endograft exclusion. Perspect Vasc Surg Endovasc Ther 2012;24:5-13. [Crossref] [PubMed]
- Markatis F, Petrosyan A, Abdulamit T, et al. Hybrid repair with antegrade visceral artery debranching: the preferred treatment option for thoracoabdominal aneurysms in high-risk patients. J Endovasc Ther 2012;19:356-62. [Crossref] [PubMed]
- Tshomba Y, Melissano G, Logaldo D, et al. Clinical outcomes of hybrid repair for thoracoabdominal aortic aneurysms. Ann Cardiothorac Surg 2012;1:293-303. [PubMed]
- Väärämäki S, Suominen V, Pimenoff G, et al. Hybrid repair of thoracoabdominal aortic aneurysms is a durable option for high-risk patients in the endovascular era. Vasc Endovascular Surg 2016;50:491-6. [Crossref] [PubMed]
- Benrashid E, Wang H, Andersen ND, et al. Complementary roles of open and hybrid approaches to thoracoabdominal aortic aneurysm repair. J Vasc Surg 2016;64:1228-38. [Crossref] [PubMed]
- Greenberg R, Eagleton M, Mastracci T., et al. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg 2010;140:S171-8. [Crossref] [PubMed]
- Guillou M, Bianchini A, Sobocinski J, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2012;56:65-73. [Crossref] [PubMed]
- Verhoeven EL, Katsargyris A, Bekkema F, et al. Ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: Results from 166 consecutive patients. Eur J Vasc Endovasc Surg 2015;49:524-31. [Crossref] [PubMed]
- Austermann M, Donas KP, Panuccio G, et al. Pararenal and thoracoabdominal aortic aneurysm repair with fenestrated and branched endografts: lessons learned and future directions. J Endovasc Ther 2011;18:157-60. [Crossref] [PubMed]
- Verhoeven EL, Vourliotakis G, Bos WT, et al. Fenestrated stent grafting for short-necked and juxtarenal abdominal aortic aneurysm: an 8-year single-centre experience. Eur J Vasc Endovasc Surg 2010;39:529-36. [Crossref] [PubMed]
- Verzini F, Loschi D, De Rango P, et al. Current results of total endovascular repair of thoracoabdominal aortic aneurysms. J Cardiovasc Surg (Torino) 2014;55:9-19. [PubMed]
- Bisdas T, Donas KP, Bosiers MJ, et al. Custom-made versus off-the-shelf multibranched endografts for endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg 2014;60:1186-95. [Crossref] [PubMed]
- Lachat M, Veith FJ, Pfammatter T, et al. Chimney and periscope grafts observed over 2 years after their use to revascularize 169 renovisceral branches in 77 patients with complex aortic aneurysms. J Endovasc Ther 2013;20:597-605. [Crossref] [PubMed]


