Fluid responsiveness raises many questions—echocardiography may be the answer
Vignon et al. (1) are to be commented for their well-designed and written manuscript on the diagnostic accuracy of echocardiographic parameters for fluid responsiveness in ventilated patients with various types of shock.
Fluid responsiveness is a common challenge for an intensivist in everyday clinical practice but so far no technique has been recognized as the most sensitive for its assessment and for the evaluation of the complex interactions of multiple organ dysfunctions and the dynamic effects of therapeutic interventions, especially in unstable hemodynamic conditions. Echocardiography has been investigated as a potential tool for fluid responsiveness since it is feasible even at bedside, useful for monitoring and able to provide real time information on heart-lung interaction, volume status and the effect of mechanical ventilation. Unfortunately available evidence on the ability of echocardiography for the assessment of fluid responsiveness is so heterogeneous for methodology (transthoracic vs. transesophageal), clinical conditions (stable/unstable, ventilated/spontaneous breathing) and etiology of disease (sepsis/other types of shock) that echocardiographic parameters may be used in the wrong context/patient with unpredictable consequences on management.
On a conceptual basis, when assessing fluid responsiveness in a single patient, it should be considered that if cardiopulmonary function cannot compensate for the increase in preload, fluid loading (even small volumes) may compromise microvascular perfusion and oxygen delivery and cause or aggravate peripheral and pulmonary edema (2-5). Thus, the first question for a front line intensivist managing a critical care patient is: in my patient can fluid responsiveness be assessed or may it be deleterious? For instance, the presence of right ventricular (RV) dilatation and RV dysfunction stands against fluid challenge which can itself worsen RV dysfunction and thus aggravate hemodynamic instability. In other terms, fluid responsiveness assessment needs a preliminary echocardiographic exam, including the evaluation of biventricular function, systolic pulmonary arterial pressure, the presence or absence of valvular disease and diastolic function.
If volume expansion is not contraindicated, which echocardiographic parameter should be used in our patient? It is conceivable that each parameter should be chosen on the basis of available evidence, type of echocardiography (transthoracic vs. transesophageal) and, if possible, etiology of disease in which this parameter has been tested (6). The methodology used for fluid responsiveness is also influenced by the patient’s clinical conditions (i.e., passive leg rising should not be performed in post abdominal surgery).
Table 1 summarizes the main investigations assessing fluid responsiveness in ventilated critically ill patients according to the type of echocardiography (transthoracic, transesophageal or both). Investigations performed in cardiac surgery and in the operating room have not been included. The contribution of each investigation on a clinical ground stems from the critical interpretation of reliability and accuracy of the single parameter investigated, taking into account not only the number of patients enrolled but also methodology used to assess fluid responsiveness (i.e., volume expansion vs. passive leg raising). To make things worse, thresholds may vary from one study to another. The major task for a clinician is probably to “adapt” methodology and parameters to the single patient, in other term to choose them accordingly to the clinical conditions of the patients and/or to the availability and/or expertise in the echocardiographic technique.
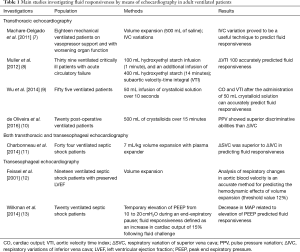
Full table
Fluid responsiveness was investigated by means of transesophageal echocardiography in septic shock in three studies published in 2001 and in 2014, respectively, all assessing different parameters. Wilkman et al. (13) observed that decreased in mean arterial pressure (MAP) due to an increased in peak end-expiratory pressure (PEEP) predicted fluid responsiveness (cut off value of ΔMAP for clinical use −8%) while Feissel et al. (12), in a small subgroup of 19 patients with septic shock and preserved LV systolic function, observed that analysis of respiratory changes in aortic blood velocity was an accurate method for predicting the hemodynamic effects of volume expansion. Different parameters were assessed by Charbonneau et al. (11) who observed that variations in superior vena cava diameters (ΔSVC) was significantly more accurate than changes in inferior vena cava width (ΔIVC) in predicting fluid responsiveness in 44 mechanical ventilated septic shock. The design of the latter study was more complex since they used both transthoracic and transesophageal echocardiography. However, in all these investigations, fluid responsiveness was defined as the volume expansion-induced increase in cardiac index was > or =15%.
In the last years, an increasing number of papers have been published on fluid responsiveness assessed by means of transthoracic echocardiography. Two are the parameters mainly investigated: subaortic velocity-time integral (VTI) and variation in inferior vena cava (IVC) diameters. Variations in VTI proved to predict fluid. responsiveness in ventilated septic shock even after 100 mL of hydroxyethyl starch infusion (1 minute) (8), and this finding was confirmed in ventilated patients also with the infusion of smaller amounts of crystalloid solution (50 mL over 15 seconds) (9).
Assessment of IVC size and its change in diameters has been investigated as a tool for fluid responsiveness (14,15), thanks to easy of acquisition and reproducibility of measurements, even if with conflicting results (8,11). However, IVC size and variations may not indicate volume status and therefore predict fluid responsiveness such as mechanical ventilation with high PEEP which itself increase IVC pressure thus increasing or leaving unaltered IVC diameter (16). Similarly, in patients with critical intra-abdominal hypertension and abdominal compartment syndrome it is conceivable to suppose that IVC variations are determined by its transmural pressure, (the pressure gradient between abdominal and intrathoracic compartments) regardless volume status. IVC width and changes are influenced by several cardiac conditions.
A cardiac condition, frequently encountered in the ICU setting, is represented by right ventricle dysfunction (independently of its etiology, ischemic or not) which leads to increase in right side filling pressure and systemic venous congestion and therefore to IVC dilatation. Obviously, in this clinical condition, fluid responsiveness cannot be assessed by IVC variations. Also cardiac tamponade is associated with an IVC fixed and dilated (due to venous congestion). This finding does not mean the absence of fluid responsiveness and should not obviously preclude volume expansion.
According to this growing body of evidence, echocardiography appears as a feasible tool for the assessment of fluid responsiveness in intensive care, since it offers multiple capabilities represented by different parameters and techniques. In this constellation of technical opportunities, the front line intensivist should orientate the decision making process using two principles. Firstly, clinical judgement guides techniques. In other words, a clinical adaptation of methodology and echocardiographic parameters should be made on the single patient, based on a preliminary echocardiographic examination, the underlying disease (i.e., sepsis vs. primary cardiac conditions), the actual clinical conditions (i.e., mechanical ventilation, abdominal surgery, atrial fibrillation) and finally the technique (transthoracic vs. transesophageal echocardiography) the physician is more skilled and expert. All these factors deeply affect the usefulness of the fluid responsiveness test in the single patient. Secondly, the results of the echocardiographic assessment of fluid responsiveness (that is responder or not responder) should be integrated with other findings, such as indexes of hypoperfusion. A strict echocardiographic monitoring of the patient should then follow since the condition of “fluid responder” may be time-limited and the identification of the switch to “non-responder” is crucial for avoiding fluid overload.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vignon P, Repessé X, Bégot E, et al. Comparison of Echocardiographic Indices Used to Predict Fluid Responsiveness in Ventilated Patients. Am J Respir Crit Care Med 2017;195:1022-32. [Crossref] [PubMed]
- Rivers EP, Jaehne AK, Eichhorn-Wharry L, et al. Fluid therapy in septic shock. Curr Opin Crit Care 2010;16:297-308. [Crossref] [PubMed]
- Cordemans C, De Laet I, Van Regenmortel N, et al. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care 2012;2:S1. [Crossref] [PubMed]
- Polderman KH, Varon J. Do not drown the patient: appropriate fluid management in critical illness. Am J Emerg Med 2015;33:448-50. [Crossref] [PubMed]
- Perner A, Vieillard-Baron A, Bakker J. Fluid resuscitation in ICU patients: quo vadis? Intensive Care Med 2015;41:1667-9. [Crossref] [PubMed]
- Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care 2016;6:111. [Crossref] [PubMed]
- Machare-Delgado E, Decaro M, Marik PE. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intensive Care Med 2011;26:116-24. [Crossref] [PubMed]
- Muller L, Bobbia X, Toumi M, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care 2012;16:R188. [Crossref] [PubMed]
- Wu Y, Zhou S, Zhou Z, et al. A 10-second fluid challenge guided by transthoracic echocardiography can predict fluid responsiveness. Crit Care 2014;18:R108. [Crossref] [PubMed]
- de Oliveira OH, Freitas FG, Ladeira RT, et al. Comparison between respiratory changes in the inferior vena cava diameter and pulse pressure variation to predict fluid responsiveness in postoperative patients. J Crit Care 2016;34:46-9. [Crossref] [PubMed]
- Charbonneau H, Riu B, Faron M, et al. Predicting preload responsiveness using simultaneous recordings of inferior and superior vena cavae diameters. Crit Care 2014;18:473. [Crossref] [PubMed]
- Feissel M, Michard F, Mangin I, et al. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 2001;119:867-73. [Crossref] [PubMed]
- Wilkman E, Kuitunen A, Pettilä V, et al. Fluid responsiveness predicted by elevation of PEEP in patients with septic shock. Acta Anaesthesiol Scand 2014;58:27-35. [Crossref] [PubMed]
- Zhang Z, Xu X, Ye S, et al. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol 2014;40:845-53. [Crossref] [PubMed]
- Barbier C, Loubières Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 2004;30:1740-6. [Crossref] [PubMed]
- Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med 2016;42:1164-7. [Crossref] [PubMed]


