Pleural and peritoneal mesotheliomas in the Friuli Venezia Giulia register: data analysis from 1995 to 2015 in Northeastern Italy
Introduction
Malignant mesothelioma (MM)—a tumor arising, more commonly, from the mesothelial lining of the pleura and the peritoneum—has been recognized as an industrial disease since 1960 when the strong causative link with asbestos exposure was confirmed (1). The connection of mesothelioma with asbestos exposure is well established with an aetiological fraction above 80% (2). Indeed, incidence of the disease prior to the widespread commercial production of asbestos was rare (3). In the 19th and 20th centuries, asbestos was regarded as safe and widely used in a large number of industries with minimal control of exposure (4). There is convincing evidence that MM is associated with occupational exposure to all commercial forms of asbestos (5).
Although most cases of mesothelioma show a history of asbestos exposure at work, there are a proportion of cases that may result from both household exposure (family of asbestos workers) and environmental exposure (air pollution from nearby asbestos industry or exposure in buildings containing asbestos). Non-occupational exposures of this type were found to account for 8.3% of cases in the period 1993–2001 in Italy (6), but have been implicated in up to 30% of current presentations in the United States (7).
The incidence of pleural mesothelioma has shown a consistent increase in many industrialized countries for several decades (8). In recent years in some countries a deceleration or leveling off of mesothelioma rates has been observed (9), whereas in other countries the incidence is still expected to rise until 2020 (10). The highest incidence rates (about 30 cases per million of population) are reported from Australia and Great Britain (11). A high incidence rate (about 23 cases per million of population) may also be estimated for The Netherlands (12). In the US, analyses of the Surveillance, Epidemiology, and End Results (SEER) Program database estimate 2,500–3,000 cases per year, predominantly in elderly men. Furthermore the SEER incidence data suggest a plateau and subsequent decline in new mesothelioma cases since the years 2000-2005 (13). In French, after a regular increase since 1980, the national incidence of pleural mesothelioma in men remained rather stable between 1998 and 2005 with a slight falling trend (1.11 vs. 0.93 per 100,000 person-years). For women, the incidence rate increased during the same period from 0.18 to 0.29 per 100,000 person-years (14). Exposure mapping within countries reveals high regional variability in incidence and mortality. In Italy mortality from pleural cancer among men increased in the period 1970–1999 from 1.64 to 3.22 per 100,000 person-years (15) with variations of about 40 times from one Province to another, reflecting the location of using asbestos industries (16).
Trends in peritoneal mesothelioma among men and women are not as well described as trends for pleural mesothelioma. In an analysis of 50 European and USA populations, the incidence rates of peritoneal mesothelioma in men were one order of magnitude lower than those of pleural mesothelioma. Age-standardized incidence rates among men range from 0.5 to about 3 cases per million populations. In most populations, rates among women are in the range 0.2–2 per million and are lower than in men (17).
Up to the end of the 1980s, Italy was the second largest asbestos producer in Europe, after the Soviet Union, and the largest in the European Community (18). Italy produced 3,748,550 tons of raw asbestos after World War II. Production rose exponentially up to the mid-1970s, reaching its peak between 1976 and 1979 at about 160,000 tons/year and decreased after 1987. Italy produced almost exclusively chrysotile; crocidolite was purchased from Australia and South Africa (15). In Italy, the first regulation on the use of asbestos was passed in 1965 and mainly aimed at reducing the risk of asbestosis. No threshold limit value was introduced, but the regulations recommended that each employee exposed to asbestos should have a specific medical examination before the beginning of such exposure and periodic medical examinations at intervals not >1 year while exposure continued. In 1986, limitations to the use of crocidolite were enforced and asbestos spraying was prohibited. The first threshold limit values were introduced in 1986 [0.2 fibers/mL (f/mL) crocidolite, 0.5 f/mL amosite, 1 f/mL other forms of asbestos] and subsequently reduced in 1991 (1 f/mL chrysotile, 0.2 f/mL other forms of asbestos and mixtures containing chrysotile) and 1992 (0.6 f/mL chrysotile). Asbestos was definitively banned in 1992. In Friuli Venezia Giulia, besides the legal restrictions and the official ban, measures to reduce exposure in some workplaces, such as shipyards and ports, were adopted in the mid-1970s, when the use of sprayed crocidolite in the Trieste-Monfalcone shipbuilding and repair area as well as the manual handling of asbestos’ jute sacks among dock workers markedly decreased.
Nevertheless, the annual number of deaths from mesothelioma in Italy has continued to rise. This can be attributed to the long latency period—time from first exposure to diagnosis—associated with mesothelioma. Mesothelioma latency is long but it is also highly variable, and can range anywhere from 13 to 70 years (19). Few studies have investigated this variability in detail. Differences in mesothelioma latency that have been observed, for example, differences by occupation (20), gender (21), and source of exposure (22), are often attributed to differences in the intensity of exposure to asbestos. There is some evidence that disease latency has an inverse relationship with duration or degree of asbestos exposure. Asbestos insulation workers (23) and those who develop asbestosis (24) tend to have experienced greater exposure to asbestos than others, and women are often thought to have had historically lower exposures than men (25). Thus, it is expected that workers employed in occupational settings with heavy asbestos exposure, males, and those with asbestosis would have shorter latencies than other asbestos workers.
Since 1995, the Friuli Venezia Giulia Mesothelioma Register, included in the network of the Italian National Mesothelioma Register (ReNaM), records the incident cases of MM in the Region, an industrial area in Northeastern Italy with a history of extensive occupational asbestos exposure, mainly due to the existence of several shipyards in the Trieste-Monfalcone district.
The present study aims (I) to assess the evolution of pleural and peritoneal mesothelioma incidence in Friuli Venezia Giulia, based on the Regional Mesothelioma Register data; (II) to evaluate the impact of historic trends in asbestos use on observed number of mesothelioma cases and (III) to investigate the latency period among asbestos workers, paying attention to potential indicators of intensity of asbestos exposure such as industry sector, gender and presence of asbestosis.
Methods
Data collection
The Friuli Venezia Giulia Mesothelioma Register provided data from the districts (Friuli Venezia Giulia is subdivided into 4 districts: Trieste, Gorizia, Udine, Pordenone) involved in the National Mesothelioma Surveillance Program. Local data were checked and standardized before inclusion in a common database. The Friuli Venezia Giulia Operative Regional Centre (COR) currently collects incident MM cases from health care institutions (especially pathology and histology units, pneumology and chest surgery wards), consults hospital discharge records and death certificates, analyzes the pathology diagnosis and classifies cases according to diagnostic certainty achieved (certain, probable, possible) (22). Data on occupational and residential history together with lifestyle habits were reported directly from the subjects or their relatives using a standardized questionnaire/interview administered by an occupational physician. Exposure to asbestos was classified as occupational (certain, probable, possible), household, environmental, hobbies, unlikely or unknown, following the National Guidelines (22). Certain occupational exposure was attributed to the subjects whose work had involved the use of asbestos or materials containing asbestos; probable occupational exposure to the subjects who had worked in a firm where asbestos was certainly used, but whose exposure could not be documented; possible occupational exposure to the subjects who had worked in a firm referring to an economic sector where asbestos had been used.
The database of Friuli Venezia Giulia Register was able to identify all cases of MM reported among residents and diagnosed between 1995 and 2015. Within this period, incidence cases active research could not be considered completed (collection of incidence data for the period 2014–2015 is still ongoing). Exposure information and medical data were reviewed (by occupational physicians) in all cases included for analysis. The diagnosis of mesothelioma was based on the pathology report, including immunohistochemical staining documenting the presence and location of mesothelioma. For each case, data on asbestos bodies, pleural plaques and asbestosis at autopsy, when performed, were recorded. Demographic data, occupational history, and personal and family health history were acquired from the questionnaire/interview. For each job, the duration, description and occupational code were recorded together with frequency of direct or bystander asbestos exposure. There was no information available about air measurement (fibers/cm3) at the workplace for any of the subjects.
Analysis
Briefly, incidence rates for pleural and peritoneal mesotheliomas, stratified by sex, were calculated for 3-year periods by dividing the number of cases by the number of person-years in each period, derived from the yearly age distributions of the Friuli Venezia Giulia population from 1995–2015. Standardized incidence rates were calculated by the direct method using the resident Italian population database in the year 2001 as the reference and were expressed for 100,000 persons. Demographic variables and medical data were examined and compared for pleural versus peritoneal mesothelioma patients (males and females). Significance of differences between groups was determined by t-test or Chi-square test. All analyses were also conducted separately for pleural and peritoneal mesothelioma cases with occupational asbestos exposure.
Latency is here defined as the time elapsing between first exposure to asbestos and diagnosis. Latency time analysis was restricted to mesothelioma cases with occupational asbestos exposure. Variables of interest were gender, anatomical site, industry sector, year of first exposure, age at first exposure, duration of exposure, year of diagnosis and presence of asbestosis. The date of first occupational exposure (certain, probable and possible) to asbestos was taken to be the date of first exposure as recorded on the standardized questionnaire/interview. Year of first exposure and age at first exposure were derived from the date of first exposure as defined above. Duration of exposure was calculated from the date of first exposure to the date of last exposure. Cases were assigned to a specific economic sector considering the whole occupational history and the industry reported most often was used. Statistical associations between independent variables and latency were initially evaluated using ANOVA. Subsequently, their association with latency was tested in a multivariate generalized model adjusted for gender, anatomical site, year of first exposure, duration of exposure and year of diagnosis. All statistical analyses have been carried out with SPSS software (ver. 20.0), with the level of significance set at P<0.05.
Results
Demographic and clinical data
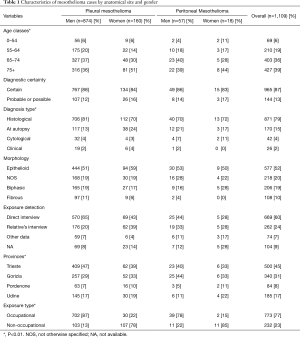
Between 1995 and 2015, 1,109 patients (931 males, 178 females) have been recorded by the Friuli Venezia Giulia Mesothelioma Register (Table 1). Among these 1,034 (93%) were certified as pleural mesothelioma and 75 (7%) as peritoneal mesothelioma. In the pleural group, 85% [874] were men and 15% [160] were females; men were diagnosed at an earlier age compared to women (70.6 vs. 74.0 years, respectively; P<0.01). About two-thirds of pleural cases (73% of men and 81% of women) were in the age over 65 years whereas only 6% of all pleural cases were younger than 54 years. In the peritoneal group, 57 (76%) were males and 18 (24%) were females. The distribution of all peritoneal cases across age was very similar to the age distribution among pleural cases. In the peritoneal group, the mean age at diagnosis was 71.4 years without statistical difference between males and females. Among women, the mean age at diagnosis for peritoneal cases was slightly younger than for pleural cases (71.5 vs. 74 years), but this difference was not statistically significant. Among pleural cases, the age at diagnosis for men was significantly younger than for women (P<0.01). On the other hand, among peritoneal cases there was no difference in the age at diagnosis between two genders. More than 80% [965] of all mesothelioma cases were diagnosed as certain, and slightly less than 15% [144] of all cases as probable or possible. The diagnosis was based upon tissue biopsy in most cases (79%) and 15% underwent post-mortem examination. Immunohistochemistry was performed in 785 (71%) cases. Among pleural mesotheliomas, subjects with a diagnosis from autopsy were significantly more frequent among women than among men (24% vs. 13%; P<0.01). There was no significant difference in the distribution of histological type between pleural and peritoneal tumors as well as between males and females. The 1109 mesotheliomas consisted of 577 epithelial (52%), 206 biphasic (19%), 108 sarcomatoid (10%) and 218 cases where the histological type was not recorded (20%). According to the data collected by the Register, the largest number of cases (75.7%) was recorded in the districts of Trieste and Gorizia—an area with a developed shipbuilding and repair industry. The smallest number of cases (24.3%) was registered inland.
Full table
Incidence
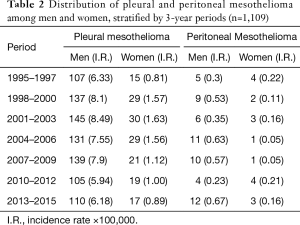
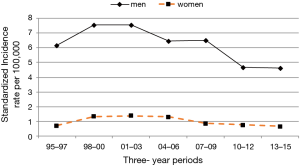
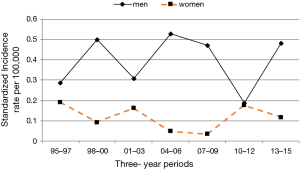
From 1995 to 2015, in Friuli Venezia Giulia the annual number of pleural mesothelioma varied between 34 and 64 cases with an average of approximately 49 cases per year. In the same period, the annual number of peritoneal mesothelioma varied from 1–6 cases per year with an average of about 4 cases per year. Table 2 presents the incidence rates (per 100,000 persons) of pleural and peritoneal mesothelioma by 3-year periods among men and women. Among men the incidence of pleural mesothelioma increased from 107 cases (6.33 per 100,000) to 139 cases (7.90 per 100,000) between 1995 and 2009, with a slight falling trend thereafter. Among women the incidence remained rather stable between 1995 and 2015, with the highest incidence rates from 1998 to 2006. In men, the incidence rate of peritoneal mesothelioma varied from 0.30 to 0.67 (per 100,000) with the highest incidence rates around 2004 and 2015. In women, the incidence rate remained stable with a falling trend between 2004 and 2009 (0.05 per 100,000). Standardized incidence rates have been estimated by the direct method using the resident Italian population database in the year 2001. The direct standardization had a small effect on the incidence rates. Rates of pleural mesothelioma were higher among men than among women across all years. In men, incidence rates of pleural mesothelioma increased rapidly during the period 1995–2003 and remained rather stable during 2004–2009 with a slight falling trend thereafter (Figure 1). Incidence rates were stable over the years among women. Rates were similar between men and women younger than 54 years, but then diverged as rates began to increase more rapidly with age among men. The incidence rate of peritoneal mesothelioma was higher among men than women during the overall period, but this difference in incidence was less pronounced during the most recent period 2010–2015 (Figure 2). In women the highest incidence rates were reached before 1998 and after 2010. The incidence rate in men, after a sudden drop during the period 2010–2012, was constant. Although the rates of pleural mesothelioma decreased, the number of mesothelioma cases diagnosed during three-year periods remained the same.
Full table
Exposure and occupational data
For all cases, the modalities of asbestos exposure have been defined in relation to the occupational, familial, residential and leisure history. Direct and indirect interviews were 67% and 26%, respectively, of all subjects with modalities of exposure ascertained. In 74 cases (7%) exposure was defined by documented working files. Information about exposure circumstances were not available for 104 (9%) cases (i.e., questionnaires could not be administered because of the poor health of the patients). Asbestos exposure was unlike or unknown (i.e., questionnaires reported an incomplete job and/or residential/familial history) for 197 (18%) cases. An occupational asbestos exposure has been found in 70% [773] of the subjects whereas 3% (33 females and 2 males) presented an exposure due to the cohabitation with someone (generally the husband or the father) occupationally exposed. The distribution by exposure modalities was particularly different between men and women, and the identification of the source of exposure was more difficult for women. The majority of occupational cases were men, whereas the majority of non-occupational cases (individuals with household or with unknown/unlikely exposure) were women. Of all men [931], 80% [741] had a history of occupational asbestos exposure, in contrast with only 18% [32] of women (P<0.01). Men with pleural mesothelioma were far more likely to have a history of occupational asbestos exposure compared to men with peritoneal mesothelioma P<0.01) (Table 1). Restricting the analysis to subjects who had been occupationally exposed to asbestos, 702 men and 30 women developed pleural mesothelioma while 39 men and 2 women developed peritoneal mesothelioma (Table 3). Occupational exposure did not appear to influence the histological type. The presence of pleural plaques and asbestosis was identified at autopsy, performed in 397 men and in 15 women. More than 60% [246] of men had pleural plaques and 50 had asbestosis as well. At autopsy, asbestosis did not find in any woman whereas pleural plaques were present in 7 women. In both genders pleural plaques were often calcified. Asbestos body count was performed in 135 cases (129 males, 6 females). Among pleural cases, 29% had high concentrations of asbestos bodies (>10,000 per gram dry lung tissue), as 57% of the peritoneal cases. Eighty percent of all cases were registered in the districts of Gorizia and Trieste (P<0.01 for pleural mesotheliomas). The exposure setting with the highest number of cases was shipbuilding [277], followed by construction [94] and transportation/ports [93]. Metal worker, laborer, welder, carpenter/joiner, insulator, electrician were the most commonly identified occupations. Among women, the most common exposure setting was the textile, followed by the service industry. Female cases had a significant shorter mean duration of exposure compared with male cases (14 vs. 22 years; P<0.01). There was no difference in duration of exposure between men with pleural versus peritoneal mesothelioma. In the whole occupationally exposed group, the mean year of first exposure was 1956 and the mean age at first exposure was 21 years, without statistical difference between pleural and peritoneal mesotheliomas as well as between males and females. Within the occupational group, the higher number of cases was registered among those born from 1930 to 1949, whereas the lower number of cases was found among those born before 1920 or after 1950.

Full table
Latency
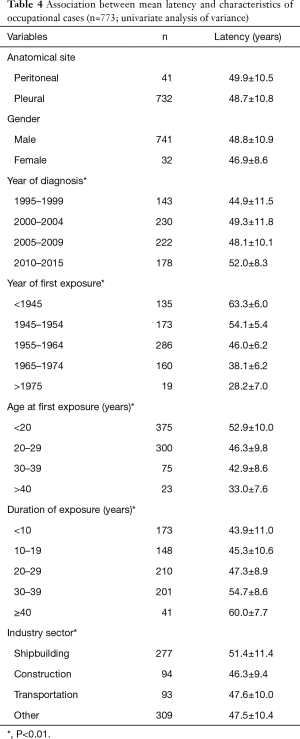
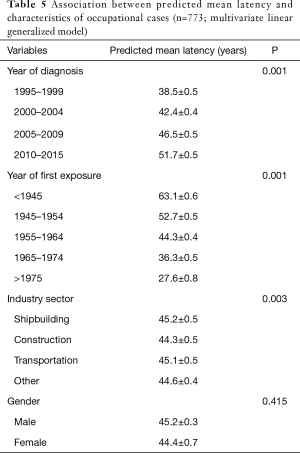
As described above (see Methods), the relationship between latency time and the variables of interest was analyzed (Table 4). Year of first exposure, age at first exposure and duration of exposure were all statistically significantly associated with latency (P<0.01). The mean latency decreased with increasing year of first exposure and age at first exposure, and increased with duration of exposure. Women had a shorter latency than men, but this difference was not statistically significant. There was no statistical difference in the latency for pleural versus peritoneal cases. Furthermore, there was no statistical difference in latency comparing those with and without asbestosis (51 vs. 50 years, respectively). Latency increased significantly with the year of diagnosis: latency time among cases diagnosed in 1995 and 2015 was 44.9 and 52 years, respectively. Limiting the analysis to the main industry sectors (shipbuilding, construction, transportation/ports, “other” industry) there was evidence of a significant association between industry sector and mesothelioma latency. Workers in the construction industry had an average shorter latency than workers in shipyard (46.3 vs. 51 years, respectively; P<0.01). When the variables of interest were included in the multivariate generalized model, the association between latency and year of first exposure, year of diagnosis and industry sector were confirmed (Table 5).
Full table
Full table
Discussion
This report updates mesothelioma incidence rates in Friuli Venezia Giulia on the basis of data from the Mesothelioma Register during the period 1995–2015. The results of the present study suggest that in men after an increase during the period 1995–2003, the incidence of pleural mesothelioma has leveled off until 2009 with a slight decrease during 2010–2015. For women, standardized incidence rates of pleural mesothelioma increased from 1995 to 2006 and then remained rather stable. The incidence rate of peritoneal mesothelioma in men was more or less constant over the study period, while rates among women increased slightly during 2010–2015. Both for pleural and peritoneal mesothelioma, the incidence rate among men was higher than among women during the overall period. Incidence rates can be influenced by several factors, including the size or overall age of the population. If the size or overall age of the population or both increased, the rate may go down, even if the number of cases stayed the same or increased during that time (26). This study shows that both in men and in women the number of pleural cases remained level during 3-year periods although in men the rate of mesothelioma incidence decreased in the recent years. However, it is underlined that the collection/transmission of incidence data over the last period [2014–2015] is still ongoing. Thus, a decrease in the pleural mesothelioma rate does not necessarily imply that there were fewer pleural mesothelioma cases in the recent years.
Mesothelioma incidence rates were highest among older men and women who were probably exposed to asbestos before efforts were undertaken to limit exposure. Among men, the distribution of all peritoneal cases across age was very similar to the age distribution among pleural cases. As most cases of pleural mesothelioma can be attributed to exposure to asbestos (27), this result suggests that the cases of peritoneal mesothelioma in men primarily reflect the effects of historical exposure to asbestos. Among women, a higher proportion (11%) of peritoneal cases were diagnosed at a younger age (<54 years) than pleural cases (6% in both genders younger than 54 years) and also at a younger age than men peritoneal cases (4%). Because mesothelioma is rare in this age group, recent evidence suggests that genetic susceptibility is another factor for peritoneal mesothelioma development (28). Several studies reported attributable fractions for asbestos and peritoneal mesothelioma of 58–75% among men and 23–33% among women (27,29). The Authors point to a more limited role of occupational exposure to asbestos in the aetiology of peritoneal mesothelioma than for pleural mesothelioma, especially among women. These findings support our data: 68% of men and only 11% of women with peritoneal mesothelioma had a history of occupational asbestos exposure. The less strong association between asbestos exposure and peritoneal mesothelioma might also explain the relatively low correlation between peritoneal and pleural mesothelioma incidence rates (17). The incidence rates of peritoneal mesothelioma among men in our study were one order of magnitude lower than those of pleural mesothelioma. A comparable analysis among women resulted in an even weak correlation until 2010. The modest correlation between pleural and peritoneal mesothelioma rates might also derive from bias in diagnostic procedures (17). Studies have shown in the past that ovarian and gastrointestinal tumors may be misdiagnosed as peritoneal mesotheliomas and vice versa (30). A small proportion of misclassification of ovarian cancer may have a great impact in the incidence of peritoneal mesothelioma among women (31). Furthermore, given the strong association between asbestos and mesothelioma, knowledge of previous exposure might influence diagnostic accuracy; if this is the case, a diagnosis of peritoneal mesothelioma would be more frequently made for a patient with recognized past asbestos exposure than for a patient with a similar clinical presentation but without history of asbestos exposure (17). However, recent immunohistochemical markers have shown good sensitivity and specificity to distinguish both peritoneal mesothelioma from serous papillary ovarian and peritoneal carcinoma as well as pleural mesothelioma from pulmonary adenocarcinoma (32). In the current study, of all peritoneal cases, 91% of males and 89% of females had a histological diagnosis obtained by biopsy or autopsy; thus, among these patients the risk of misclassification should be negligible. Immunohistochemistry tests were performed in almost 80% of female peritoneal cases further reducing the risk of a wrong diagnosis.
The analysis of mesothelioma cases distribution by geographical area in Friuli Venezia Giulia showed that the areas with the highest number of male cases were those areas containing ports and dockyards, such as Trieste and Gorizia. The link between the heavy asbestos exposure and the shipbuilding industry is well known (33). Asbestos was used widely in insulation and workers were exposed to it during building fitting and refurbishment and in ship breaking activities (11). At a national level, mesothelioma death distribution exactly reflects the location of using asbestos industries (8). Similarly, in Sweden and United Kingdom marked differences have been observed from one area to another, with the highest mesothelioma incidence rates in the counties characterized in the past by high shipbuilding activity (11,34). In Croatia, the age standardized incidence rate among men resident in the coastal area (26.6 per million), where shipyard and an asbestos-cement factory are located, was significantly higher than among men of inland area (6.9 per million) (35). The results of the geographical analysis also revealed that the number of female cases were higher in those areas with a high number of male cases, suggesting a common asbestos exposure. While men were primarily exposed to asbestos through occupation, women could be exposed in other ways. Examples of these frequently non-occupational exposures include domestic exposure from cohabitation with an asbestos worker and handling of his/her work clothes, air pollution from nearby asbestos industry, or exposure to asbestos in place (buildings containing asbestos) (36). This is supported by the results of the present study in which a significantly greater proportion of non-occupational cases (individuals with household or with unknown/unlikely exposure) were women. In all, about 80% of female cases did not report any occupational exposure but more than 20% of these cases were attributed to living with an asbestos worker. This would imply that more than half of non-occupational female cases could be presumably due to environmental asbestos exposure or unreported asbestos exposure in activities that we had classified as low risk (e.g., office, educational, health care) or to other factors. Report of these data is important as non-occupational subjects are more likely to be under-recognized than those with occupational exposure because of recall and gender bias (37). In this study a significant number of female cases (28%) were diagnosed at autopsy. Moreover, in many cases, if not all, asbestos exposure was based on patient or relatives’ interview that can be flawed by memory. Most people report their own or their parent’s occupations correctly many years later (38), but recall of past asbestos exposure shows poor reproducibility at re-interview (39). A great proportion of male cases, as would be expected, had some direct or bystander occupational exposure. As noted previously, in this Region men worked to a much larger extent in industries that used asbestos than women. The role of occupational exposure to asbestos in causing peritoneal mesothelioma has been confirmed in two community-based studies (27,40). According to the Authors, the occurrence of peritoneal mesothelioma has been linked to specific industry with high exposures, such as insulation companies and shipyards, from the use of crocidolite and amosite. Our data support these findings: a small number of females with occupational exposure developed peritoneal mesothelioma and no women at autopsy, when performed, had asbestosis. Pleural plaques were present in almost half and in more than half of female and male cases with occupational exposure, respectively, whereas asbestosis was present in about one-eighth (13%) of male cases, suggesting that, in general, pleural plaques and mesothelioma require a lower dose of asbestos than does asbestosis (41).
Examining the occupational group, women had a significant shorter duration of exposure compared with men. There was no statistical difference in duration of exposure between pleural and peritoneal cases. Furthermore, there was no difference in the year of first exposure and in the age at first occupational exposure between genders as well as between pleural and peritoneal cases. Within the occupational group, the analysis by birth cohort showed the lower number of cases in younger birth cohorts, in contrast with the higher number of cases in older birth cohorts, such as those born between 1930 and 1949. As mesothelioma depends on the latency period, the results of our analysis suggest that most manual workers started working at 15–20 years of age, when asbestos exposure was common, and were occupationally exposed first at that age. Additionally, the low number of cases in young ages would indicate that the ban in 1992 and several restrictions on the use of asbestos in the mid-1970s, have decreased the risk of mesothelioma in those who started their working career during or after this period. Since 1975 in Friuli Venezia Giulia, the implementation of the first safety measures regarding asbestos exposure at workplace (e.g., limitation in the use of sprayed crocidolite in shipyards) would be an explanation for the slight decreasing in incidence rates observed in the last years. It seems that the effects of the restrictions have already been observed in some countries, like Sweden (34), or in some sectors, such naval dockyard in the UK (42). However, as suggested from a recent study, the total effect of the ban can be evaluated only by cohorts starting their working career in the 1990s, i.e., those born 1970 or later (43).
It is currently stated that mesothelioma latency (time from first exposure to diagnosis) is long, with a peak at 30–40 years. Investigators in New South Wales, Australia, reported an average latency of 42.8 years for cases diagnosed between 1972 and 2004, without gender difference. Longer latency periods were evident in more recent diagnoses (44). A study from Italy found a mean latency of 44.6 years in 2,544 cases diagnosed in the period 1993 to 2001 with shorter latency in those cases with occupational exposure (24). In the present study we found a prolonged latency period (48.7 years) for the occupational group, consistent with the results of a previous study which reported the same latency (48.8 years) for 400 pleural mesotheliomas investigated in the Trieste-Monfalcone area (45). Some data indicate that an inverse relationship exists between intensity of exposure and length of the latency period (8). Thus, the association between mesothelioma latency and potential indicators of intensity of asbestos exposure (e.g., industry sector, gender, presence of asbestosis) was analyzed. Gender, presence of asbestosis and mesothelioma site did not show clear associations with mesothelioma latency. On the other hand, year of first occupational asbestos exposure, year of diagnosis and industry sector were associated with mesothelioma latency. In examining the latency for pleural cases compared with peritoneal cases, we were not able to detect significant differences, in keeping with the findings of other studies (21). Additionally, we found no statistical difference in latency between male and female cases occupationally exposed. A US study reported that women had a longer latency than men, due to their mainly non-occupational asbestos exposure (21). However, the source of exposure in this latency analysis was occupational for both genders and this can be an explanation for our results. Furthermore, other sources cannot be ruled out. There was no association between asbestosis and mesothelioma latency, which was in agreement with other studies (21). As those with asbestosis are thought to have experienced more intense asbestos exposure, this result would seem contrary to the intensity hypothesis. It is probably that, in this study, even individuals without asbestosis had heavy enough asbestos exposure to prevent detection of differences in latencies. As expected, year of first exposure and latency were closely linked: the latency decreased with increasing year. The association between year of diagnosis and mesothelioma latency was statistically significant. The increase in latency time by year of diagnosis could be due to some reduction of the intensity of asbestos exposure in occupational settings before the asbestos ban (22). Occupation is probably the strongest indicator of intensity of asbestos exposure and has the most consistent association with latency in the literature (25). Limiting the latency analysis to the main industry sectors—shipbuilding, construction and transportation (ports)—we found that construction workers had a shorter latency than the average of occupational cases. Although occupation in the shipbuilding and repair industry has determined very high exposures in some jobs, and that it is associated with the highest mesothelioma incidence areas in Italy (22), latency in this sector was longer than the average. A prolonged latency among shipyard workers in the Trieste-Monfalcone district has been reported by Bianchi et al. (45) and it can be explained considering the occurrence of competing diseases (asbestos lung cancer and asbestosis) in the occupational group with most heavy exposure levels (20). The ideal when someone investigates the intensity hypothesis would be to have quantitative measures of exposure in the workplace, but these were not available.
Several potential sources of bias in this study must be considered. The investigation of asbestos exposure modalities was based on information provided by patients that may have given inaccurate histories of exposure or by a relative (if the patient was dead) with a loss in the quality of information. Although in a large amount of cases the exposure could be ascertained through documents obtained from the Italian Social Security Institute, it is necessary to underline that it is not easy to identify the onset of asbestos exposure. In this analysis the start of asbestos exposure was defined as the year in which the subject began the first job, considered as related to the exposure. As a matter of fact, the beginning of a work period cold not exactly correspond to the beginning of exposure to asbestos and it could lead to an overestimation of latency time. In addition, the possible presence of competitive causes of death, the retrospective design of the study and the incomplete cohort analysis (cases reported in this study were collected in recent years and, hence, cases with relevant past exposure and short latency could be missing) induce a possible bias in the statistical inference about differences in latency.
In summary, the main results of the present study evidence that, firstly, the trends in the incidence of pleural mesothelioma in both men and women, in Friuli Venezia Giulia, have been strongly influenced by occupational exposure to asbestos and, potentially, by the implementation of safety regulations at work since 1975. In contrast, trends of peritoneal mesotheliomas, especially among women, have not been influenced by the trends in occupational exposure, but might reflect improved diagnostic accuracy during recent years and presumably the higher life expectancy of women in the Friuli Venezia Giulia population. Secondly, our data provide some evidence of a relationship between several indicators of intensity of asbestos exposure (occupational setting, year of first occupational exposure, year of diagnosis) and length of latency, despite the absence of historical fiber concentration measurements in different Friuli Venezia Giulia industries. These results raise the problem that, as asbestos is present in many buildings and homes constructed before the ban [1992], asbestos removal workers, in particular, but also other workers in any trade may be at risk when asbestos is disturbed during construction maintenance and/or refurbishment work. This accidental or inadvertent exposure to asbestos is a cause of great concern, and highlights the need to find a more accurate method of assessing occupational asbestos exposure and a reliable health surveillance tool (with high specificity and sensitivity) for the early detection of asbestos-related diseases (4). To date, control of exposure to asbestos, in particular at workplace, remains the main approach for the prevention of mesothelioma (17).
Acknowledgements
The authors wish to thank Dr. Renata De Zotti, Dr. Anna Muran, Dr. Donatella Calligaro, Dr. Antonella De Toni, Dr Paolo Barbina, Dr. Laura Fassari, Dr. Barbara Alessandrini, Dr. Andrea Camilli, Dr. Claudia D’Alessandro and Dr. Concetta Sarto for their contributions in providing the data utilized in this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;17:260-71. [PubMed]
- McDonald JC, McDonald AD. The epidemiology of mesothelioma in historical context. Eur Respir J 1996;9:1932-42. [Crossref] [PubMed]
- Strauchen JA. Rarity of malignant mesothelioma prior to the widespread commercial introduction of asbestos: the Mount Sinai autopsy experience 1883-1910. Am J Ind Med 2011;54:467-9. [Crossref] [PubMed]
- Sen D. Working with asbestos and the possible health risks. Occup Med (Lond) 2015;65:6-14. [Crossref] [PubMed]
- WH Organization, WPE Scheme. Elimination of asbestos-related diseases. In: Geneva World Health Organization. Geneva: WHO, 2006.
- Mirabelli D, Cavone D, Merler E, et al. Non-occupational exposure to asbestos and malignant mesothelioma in the Italian National Registry of Mesotheliomas. Occup Environ Med 2010;67:792-4. [Crossref] [PubMed]
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603. [Crossref] [PubMed]
- Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health 2007;45:379-87. [Crossref] [PubMed]
- Price B, Ware A. Mesothelioma trends in the United States: an update based on Surveillance, Epidemiology, and End Results Program data for 1973 through 2003. Am J Epidemiol 2004;159:107-12. [Crossref] [PubMed]
- Kjaergaard J, Andersson M. Incidence rates of malignant mesothelioma in Denmark and predicted future number of cases among men. Scand J Work Environ Health 2000;26:112-7. [Crossref] [PubMed]
- McElvenny DM, Darnton AJ, Price MJ, et al. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond) 2005;55:79-87. [Crossref] [PubMed]
- Burdorf A, Järvholm B, Englund A. Explaining differences in incidence rates of pleural mesothelioma between Sweden and the Netherlands. Int J Cancer 2005;113:298-301. [Crossref] [PubMed]
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39:576-88. [Crossref] [PubMed]
- Le Stang N, Belot A, Gilg Soit Ilg A, et al. Evolution of pleural cancers and malignant pleural mesothelioma incidence in France between 1980 and 2005. Int J Cancer 2010;126:232-8. [Crossref] [PubMed]
- Marinaccio A, Montanaro F, Mastrantonio M, et al. Predictions of mortality from pleural mesothelioma in Italy: a model based on asbestos consumption figures supports results from age-period-cohort models. Int J Cancer 2005;115:142-7. [Crossref] [PubMed]
- Mastrantonio M, Belli S, Binazzi A, et al. La mortalità per tumore maligno della pleura nei comuni italiani, 1988-1997. Rapporti ISTISAN 2002;02/12 (in Italian).
- Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol 2007;18:985-90. [Crossref] [PubMed]
- Marinaccio A, Binazzi A, Di Marzio D, et al. Pleural malignant mesothelioma epidemic: incidence, modalities of asbestos exposure and occupations involved from the Italian National Register. Int J Cancer 2012;130:2146-54. [Crossref] [PubMed]
- Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med 1992;34:718-21. [PubMed]
- Bianchi C, Giarelli L, Grandi G, et al. Latency periods in asbestos-related mesothelioma of the pleura. Eur J Cancer Prev 1997;6:162-6. [PubMed]
- Haber SE, Haber JM. Malignant mesothelioma: a clinical study of 238 cases. Ind Health 2011;49:166-72. [Crossref] [PubMed]
- Marinaccio A, Binazzi A, Cauzillo G, et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur J Cancer 2007;43:2722-8. [Crossref] [PubMed]
- Williams PR, Phelka AD, Paustenbach DJ. A review of historical exposures to asbestos among skilled craftsmen (1940-2006). J Toxicol Environ Health B Crit Rev 2007;10:319-77. [Crossref] [PubMed]
- Jamrozik E, de Klerk N, Musk AW. Asbestos-related disease. Intern Med J 2011;41:372-80. [Crossref] [PubMed]
- Frost G. The latency period of mesothelioma among a cohort of British asbestos workers (1978-2005). Br J Cancer 2013;109:1965-73. [Crossref] [PubMed]
- Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep 1998;47:1-16,20. [PubMed]
- Spirtas R, Heineman EF, Bernstein L, et al. Malignant mesothelioma: attributable risk of asbestos exposure. Occup Environ Med 1994;51:804-11. [Crossref] [PubMed]
- Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022-5. [Crossref] [PubMed]
- Roggli VL, Sharma A, Butnor KJ, et al. Malignant mesothelioma and occupational exposure to asbestos: a clinicopathological correlation of 1445 cases. Ultrastruct Pathol 2002;26:55-65. [Crossref] [PubMed]
- Krasuski P, Poniecka A, Gal E. The diagnostic challenge of peritoneal mesothelioma. Arch Gynecol Obstet 2002;266:130-2. [Crossref] [PubMed]
- Burdorf A, Järvholm B, Siesling S. Asbestos exposure and differences in occurrence of peritoneal mesothelioma between men and women across countries. Occup Environ Med 2007;64:839-42. [Crossref] [PubMed]
- Goldberg M, Imbernon E, Rolland P, et al. The French National Mesothelioma Surveillance Program. Occup Environ Med 2006;63:390-5. [Crossref] [PubMed]
- Hillerdal G. The Swedish experience with asbestos: history of use, diseases, legislation, and compensation. Int J Occup Environ Health 2004;10:154-8. [Crossref] [PubMed]
- Hemminki K, Li X. Time trends and occupational risk factors for pleural mesothelioma in Sweden. J Occup Environ Med 2003;45:456-61. [Crossref] [PubMed]
- Curin K, Sarić M, Strnad M. Incidence of malignant pleural mesothelioma in coastal and continental Croatia: epidemiological study. Croat Med J 2002;43:498-502. [PubMed]
- Hillerdal G. Mesothelioma: cases associated with non-occupational and low dose exposures. Occup Environ Med 1999;56:505-13. [Crossref] [PubMed]
- Faig J, Howard S, Levine EA, et al. Changing pattern in malignant mesothelioma survival. Transl Oncol 2015;8:35-9. [Crossref] [PubMed]
- Berney LR, Blane DB. Collecting retrospective data: accuracy of recall after 50 years judged against historical records. Soc Sci Med 1997;45:1519-25. [Crossref] [PubMed]
- Holmes E, Garshick E. The reproducibility of the self-report of occupational exposure to asbestos and dust. J Occup Med 1991;33:134-8. [PubMed]
- Cocco P, Dosemeci M. Peritoneal cancer and occupational exposure to asbestos: results from the application of a job-exposure matrix. Am J Ind Med 1999;35:9-14. [Crossref] [PubMed]
- Roggli VL, Sharma A. Analysis of tissue mineral fiber content. In: Roggli VL, Oury TD, Sporn TA, et al. editors. Pathology of asbestos-associated diseases. Boston: Little Brown, 2004:309-54.
- Hilliard AK, Lovett JK, McGavin CR. The rise and fall in incidence of malignant mesothelioma from a British Naval Dockyard, 1979-1999. Occup Med (Lond) 2003;53:209-12. [Crossref] [PubMed]
- Järvholm B, Burdorf A. Emerging evidence that the ban on asbestos use is reducing the occurrence of pleural mesothelioma in Sweden. Scand J Public Health 2015;43:875-81. [Crossref] [PubMed]
- Hyland RA, Ware S, Johnson AR, et al. Incidence trends and gender differences in malignant mesothelioma in New South Wales, Australia. Scand J Work Environ Health 2007;33:286-92. [Crossref] [PubMed]
- Bianchi C, Romani L, Bianchi T, et al. Latency periods in asbestos-related mesothelioma of the pleura. Med Lav 2002;93:385.


