A novel facilitated negative-pressure wound therapy for thoracic incision infection after esophagectomy
Introduction
Esophagectomy is the mainstay of treatment for malignant oesophageal disease without distant metastasis. However, high rates of postoperative morbidity remain a vexing problem. In particular, postoperative infection is the most common kind of postoperative morbidity (1,2). An anastomotic leak is a devastating postoperative infectious complication and gives rise to clinical concern, but surgical site infections receive less attention. The rate of surgical site infections continues to be unacceptably high, bringing pain and economic losses to the patient as a result of the associated morbidity and mortality (3). Although minimally invasive esophagectomy has lower wound infection rates, open surgery remains the primary treatment for local advanced oesophageal cancer (4). Therefore, the application of an effective method plays an important role in the control of surgical site infections after surgery.
Negative-pressure wound therapy (NPWT) is widely used among the surgical specialties to apply negative pressure to a wound bed to promote wound healing (5-7). The wound cavity is filled with polyurethane foam and drainage tubes. One or two suction tubes are positioned and drawn transcutaneously through the foam (8). More recently, several studies in which patients with severe intrathoracic infections were managed with intrathoracic NPWT found that NPWT efficiently controlled intrathoracic infections and preserved chest wall integrity (9-11). However, the efficacy of NPWT in the treatment of thoracic incision infection is unclear. In this study, we evaluate the effectiveness and safety of a novel facilitated NPWT in the treatment of thoracic incision infection after esophagectomy compared to conventional open wound therapy.
Methods
Patient population
Three hundred eighty consecutive patients underwent open esophagectomy for oesophageal cancer in the Thoracic Department of the First Affiliated Hospital, College of Medicine, Zhejiang University, between January 2013 and March 2016. Forty-five patients with thoracic incision infection were retrospectively reviewed. Of these patients, 25 patients were treated with NPWT and 20 were treated with open wound dressing. The indication of NPWT is wound inflammation and effusion. Surgical site infection was defined in accordance with the guidelines of the U.S. Centers for Disease Control and Prevention (12). All surgical site infections were evaluated for wound inflammation and effusion. The following information was retrieved: age, gender, smoking and alcohol intake history, body mass index, American Society of Anesthesiologists risk class, concomitant morbidities, pathologic details, intraoperative clinical data, postoperative complications, the duration of postoperative hospitalisation and wound healing and the wound treatment cost. The study was approved by the ethics committee and the Institutional Review Board of the First Affiliated Hospital, School of Medicine, Zhejiang University.
Procedure description
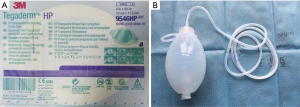
The facilitated NPWT device was used in 25 patients. The novel device includes a transparent film dressing, drainage tubing and a silica gel negative-pressure suction ball (Figure 1). The drainage tube was inserted into the wound though the infection site. The transparent film dressing was positioned around the surgical site infection with the drainage tubes extending outside. The other end of the drainage tube was connected directly to a silica gel negative-pressure suction ball to maintain a negative-pressure environment [the pressure was kept in 6.7–26.7 kPa (125–200 mmHg)]. The drainage tube and silica gel negative-pressure suction ball were fixed (Figure 2A). When the drainage fluid from the wounds ceased and the wound infections were controlled, the NPWT dressings were removed, the wounds were healed (Figure 2B). The patients were satisfied with the outcome of NPWT. The other 20 patients were treated with traditional open wound dressing. After the granulation tissue was growing well and the wound infection was controlled, the patient was discharged and the dressing was changed on an outpatient basis. Prophylactic antibiotics were routinely prescribed for all patients after surgery. In cases of infection, the empirical use of antibiotics was continued until evidence was obtained from culture and sensitivity testing.

Statistical analysis
The data were expressed as mean ± standard deviation for continuous parametric variables and were compared with the two-tailed Student’s t-test. Continuous nonparametric data were compared with the Mann-Whitney U test. For enumeration data, we applied the Pearson χ2 or Fisher’s exact test to compare the associated variables between the two groups. Values for P of less than 0.05 were considered to indicate statistical significance. All of the statistical analyses were performed using IBM SPSS statistical software version 20.
Results
Patient clinical characteristics
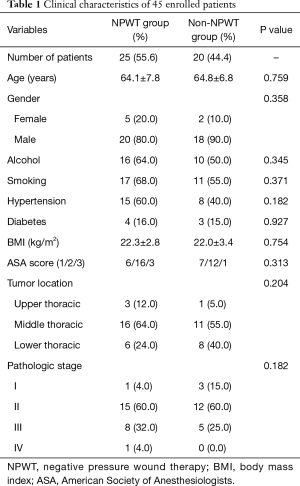
In our study, the rate of thoracic incision infection was 11.8%. All of the tumours were identified as squamous cell carcinomas after pathologic review, and all of the patients reconstructed gastric tube via the posterior mediastinal reconstruction. The clinical characteristics and intraoperative clinical data of the patients in both groups are listed in Tables 1,2. No statistically significant differences were observed between the two groups with regard to their clinical characteristics.
Full table

Full table
Results of NPWT
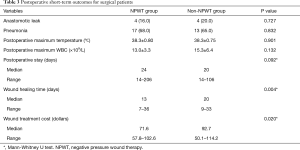
All of the incision infections were cured in the hospital or on an outpatient basis. No allergic reactions or other side effects occurred with NPWT. No patient in whom NPWT failed to cure the wound healing and converted to the open drainage system. The incidence of other major postoperative complications, including anastomotic leaks and pneumonia were comparable between the two groups (Table 3). The postoperative maximum body temperature and white blood cell count also were not statistically significant. The postoperative stay of the patients who were treated with NPWT showed no significant difference compared to the traditional treatment group (P=0.092). However, the median wound healing time was significantly shorter in the NPWT group (13 days) than in the traditional open wound dressing group (20 days; P=0.004). Five patients (3 of NPWT group and 2 of non-NPWT group) examined enterococcus faecalis from the wound fluid. Then, macrolides or fluoroquinolones antibiotics were prescribed.
Full table
Wound treatment cost
The wound treatment cost mainly included wound dressing and the installation cost of the NPWT device. The cost to install the facilitated NPWT device was only about $30. When compared with traditional open wound dressing treatment, the NPWT group was associated with lower wound treatment cost ($71.60 vs. $92.70; P=0.020).
Discussion
The wound infection rate in patients who undergo open classical surgery varies between 1.89% and 18.92% (13). In our patient set, thoracic incision infection complications were observed in 45 patients (11.8%). Classically, conventional open wound dressing therapy requires a significant period of time, involves daily changes of the wound dressings, and brings huge painful and economic loss to the patient. We design a convenient and cost-effective NPWT to control surgical site infections after surgery.
NPWT was first described by Fleischmann in 1993 (14). He described this method in patients with open fractures and achieved very good results. Since then, NPWT has been widely used to assist in the treatment of a variety of wound types (10,15). NPWT is based on continuous or intermittent application of negative pressure to remove excess fluid from the wound to prevent lacuna formation and increase the local microcirculation to stimulate the growth of granulation tissue (16). The transparent film dressing keeps the wound closed and allows observation of the wound in a timely manner, so there is no need for daily changes of the wound dressings, which reduces the doctors’ work and spares the patients pain.
The molecular mechanism for the use of NPWT for wound closure remains undefined. One possible mechanism is that the negative pressure and closed wound keep the wound stressed and hypoxic, which consequently triggers mechanoreceptor and hypoxia-mediated signalling pathways (17,18). The pathways’ cytokine and growth factor (e.g., tumour necrosis factor; interleukin-1β, -6, -8 and -10; vascular endothelial growth factor; fibroblast growth factor 2; transforming growth factor β; platelet-derived growth factor; and matrix metalloproteinases-1, -2, -9 and -13) expression stimulate angiogenesis, culminating in the remodelling of the extracellular matrix and the growth of granulation tissue, both of which promote wound healing (19).
In thoracic surgery, the use of NPWT for sternal infections, complex chest-wall reconstruction infections and severe intrathoracic infection has been reported (9-11,20,21). The results have shown that negative-pressure therapy efficiently controlled infections and preserved chest wall integrity. However, the efficacy of NPWT in the treatment of thoracic incision infection is unclear. The surgical site infections are less serious than those of the thoracic cavity, so these infections always receive less attention. Actually, the rate of surgical site infections continues to be unacceptably high, bringing great pain and economic losses to patients from the associated morbidity and mortality (3). The current standard management for surgical site infections includes open wound dressing changes until the wound is covered by granulation tissue. However, this procedure is cumbersome for patients and requires frequent dressing changes and prolonged wound healing (10). As a consequence, an NPWT technique to improve the speed of wound healing should be developed.
Classically, a medical NPWT device includes elastic sealing rubber films, a porous soft foam cushion, drainage tubes and a negative-pressure suction system (8,22). Although it has shown certain advantages over traditional open wound treatment, this device is inconvenient for surgical site infections. The cost of installing the device is relatively high, and the methods are very complex in practical operation. To solve these problems, we designed a convenient and cost-effective NPWT to control surgical site infections after surgery. The novel device includes a transparent film dressing, drainage tubing and a silica gel negative-pressure suction ball. The cost to install the facilitated NPWT device was only about $30. Although the patients who were treated with the facilitated NPWT did not have a significantly shorter postoperative hospital stay than those who had open wound dressing, this medical NPWT provided a good sealing effect, is simple in structure and convenient to use, and allows for a shortened wound healing time and reduced wound treatment cost compared with traditional open wound treatment.
The limitations of this study lie in the nature of its retrospective design and may have led to an unintended selection bias of patients and a failure to incorporate some cytokine and growth factors to research the molecular mechanism of the use of NPWT for wound healing. In addition, although we used the U.S. Centers for Disease Control and Prevention definition of surgical site infection, certain components allow for subjective interpretation of wound inflammation and effusion and other signs or symptoms of wound infection. Thus, to further evaluate this technique, prospective data collection and incorporation of some molecular factors are encouraged.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study received approval from the Ethics Committee of The First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2016059). A waiver of consent was granted for this retrospective data review.
References
- Neoral C, Horakova M, Aujesky R, et al. Infectious complications after esophagectomy. Surg Infect (Larchmt) 2012;13:159-62. [Crossref] [PubMed]
- Kataoka K, Takeuchi H, Mizusawa J, et al. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Alexander JW, Solomkin JS, Edwards MJ., et al. Updated recommendations for control of surgical site infections. Ann Surg 2011;253:1082-93. [Crossref] [PubMed]
- Sihag S, Kosinski AS, Gaissert HA., et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Comparison of Early Surgical Outcomes From The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101:1281-8; discussion 1288-9. [Crossref] [PubMed]
- Carlson GL, Patrick H, Amin AI, et al. Management of the open abdomen: a national study of clinical outcome and safety of negative pressure wound therapy. Ann Surg 2013;257:1154-9. [Crossref] [PubMed]
- Ni J, Liu H, Liu X, et al. Vacuum Sealing Drainage as Treatment of Severe Buttocks and Perianal Infection: A Case Report and Review of the Literature (Care-Compliant). Medicine (Baltimore) 2015;94:e1766. [Crossref] [PubMed]
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg 2016;103:477-86. [Crossref] [PubMed]
- Ellis G. How to apply vacuum-assisted closure therapy. Nurs Stand 2016;30:36-9. [Crossref] [PubMed]
- Saadi A, Perentes JY, Gonzalez M, et al. Vacuum-assisted closure device: a useful tool in the management of severe intrathoracic infections. Ann Thorac Surg 2011;91:1582-9. [Crossref] [PubMed]
- Begum SS, Papagiannopoulos K. The use of vacuum-assisted wound closure therapy in thoracic operations. Ann Thorac Surg 2012;94:1835-9; discussion 1839-40.
- Perentes JY, Abdelnour-Berchtold E, Blatter J, et al. Vacuum-assisted closure device for the management of infected postpneumonectomy chest cavities. J Thorac Cardiovasc Surg 2015;149:745-50. [Crossref] [PubMed]
- Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606-8. [Crossref] [PubMed]
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. [Crossref] [PubMed]
- Fleischmann W, Strecker W, Bombelli M, et al. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg 1993;96:488-92. [PubMed]
- Glass GE, Nanchahal J. The methodology of negative pressure wound therapy: separating fact from fiction. J Plast Reconstr Aesthet Surg 2012;65:989-1001. [Crossref] [PubMed]
- Li W, Ji L, Tao W. Effect of vacuum sealing drainage in osteofascial compartment syndrome. Int J Clin Exp Med 2015;8:16112-6. [PubMed]
- Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol 1997;9:707-13. [Crossref] [PubMed]
- Saxena V, Orgill D, Kohane I. A set of genes previously implicated in the hypoxia response might be an important modulator in the rat ear tissue response to mechanical stretch. BMC Genomics 2007;8:430. [Crossref] [PubMed]
- Glass GE, Murphy GF, Esmaeili A, et al. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg 2014;101:1627-36. [Crossref] [PubMed]
- Rocco G, Martucci N, La Rocca A, et al. Postoperative local morbidity and the use of vacuum-assisted closure after complex chest wall reconstructions with new and conventional materials. Ann Thorac Surg 2014;98:291-6. [Crossref] [PubMed]
- Baillot R, Cloutier D, Montalin L, et al. Impact of deep sternal wound infection management with vacuum-assisted closure therapy followed by sternal osteosynthesis: a 15-year review of 23,499 sternotomies. Eur J Cardiothorac Surg 2010;37:880-7. [Crossref] [PubMed]
- Hudson DA, Adams KG, Van Huyssteen A, et al. Simplified negative pressure wound therapy: clinical evaluation of an ultraportable, no-canister system. Int Wound J 2015;12:195-201. [Crossref] [PubMed]


