Totally thoracoscopic pulmonary anatomic segmentectomies: technical considerations
Video-assisted thoracic surgery (VATS) and thoracoscopic major pulmonary resections are accepted as a valid alternative to open surgery as it is now evident that minimally invasive surgery is beneficial in terms of reduced postoperative pain, shorter hospital stay, shorter recovery and better compliance to adjuvant chemotherapy, without compromising oncological principles (1). However few series of video-assisted pulmonary segmentectomies have been published and totally endoscopic-so-called complete VATS-segmentectomies series are even more infrequently reported (2,3). Many different techniques of thoracoscopic major pulmonary resections have been described, depending on the use of an accessory mini-thoracotomy, endoscopic instrumentation, and, video display. In the totally endoscopic approach only endoscopic instruments and monitor visualization are used. This is the technique that will be described in this article (4). By totally endoscopic we mean: (I) 100% video display; (II) no access incision and (III) only use of trocars and endoscopic instruments (5) (Figures 1,2). The aim of this article is not to discuss the oncologic validity of segmentectomies for early stage lung carcinomas but to describe and discuss some technical aspects and the results of totally thoracoscopic anatomic segmentectomies (TTAS).
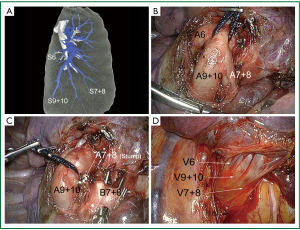
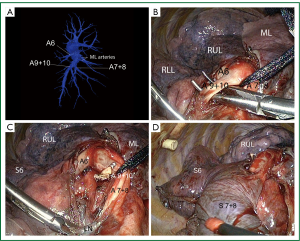
Patients and methods
From January 2008 to January 2013, TTAS was attempted in 117 patients (51 males and 66 females) ranging in age from 18 to 81 years (mean: 62 years). The indication was either a benign lesion (31 patients), a solitary metastasis (17 patients), or a suspicion of clinical stage I non-small-cell lung carcinoma (NSCLC) (69 Patients). The reason for performing a segmentectomy for an NSCLC was an impaired lung function and/or a previous history of pulmonary resection, clinical stage IA in fragile patients or carcinoid tumor.
Patients’ consent was routinely obtained. Intraoperative and postoperative data were recorded in a prospective manner into a database that was approved by our Institutional Review Board. The variables entered in the database were the following: need for conversion to thoracotomy, duration of the surgical procedure as noted on the operating room records, operative blood loss, intraoperative complications, number of collected lymph nodes and of dissected lymph node stations for patients operated on for NSCLC, duration of chest drainage, postoperative stay and postoperative complications. The types of segmentectomy are specified in Table 1.

Full Table
Technical aspects
We have previously described our technique in detail (Gossot, 2010#53). In brief, the procedure was performed under general anesthesia with split ventilation using a double-lumen endotracheal tube. Patients were positioned in lateral decubitus as for a thoracotomy. The surgeon stood anterior or posterior to the patient, depending on the segments to be resected. He usually stood posterior to the patient for right sided resections and anteriorly for left sided ones. Two monitors were used and the thoracoscope was placed on a mechanical scope holder. In a fashion similar to our technique of totally endoscopic lobectomies, we used a deflectable thoracoscope housing a distal CCD (LTF, Olympus, Tokyo, Japan) (6) connected to a high definition camera system (HDTV) (Exera II, Olympus, Tokyo, Japan). Only specifically designed endoscopic instruments for VATS major resections were used. As a rule, trocars with a diameter ranging between 3 mm (micro-instruments) and 15 mm (endostapler and retrieval bag were utilized). For lung cancer patients, intersegmental lymph nodes, when present, were analyzed by frozen section to confirm the indication for segmentectomy. Larger vessels were divided with endostaplers while haemostasis of small caliber vessels was performed with clips, with a bipolar vessel sealing device (LigaSure™, Valleylab, Boulder, CO, USA) or with a combination of both methods. The root of the intersegmental veins was preserved and used as landmark for identification of the intersegmental plane. Demarcation between the resected and preserved segments was usually made possible by gentle reventilation and adequate application of a long 5-mm lung forceps whose position was adapted according to the inflation-deflation line. The intersegmental plane was divided by a combination of bipolar sealing device (for its peripheral and thin portion) and stapling (for its central and thick portion) using 4.8 mm staples (Endo-GIA II, Covidien Autosuture, Mansfield, MA). When the remaining segment was mobile and at risk of torsion, it was anchored to the adjacent lobe with a TA endostapler. An additional radical lymphadenectomy was performed for all patients operated on for a suspicion of lung carcinoma, according to a previously described technique (7). No utility incision was used. On completion of the pulmonary resection, the specimen was wrapped into an endobag and retrieved through one of the port sites that was enlarged to a length of 2 to 4 cm, depending on the specimen size. The use of a rib spreader was never required for specimen extraction. In most cases, only 1 chest tube was placed through one of the port site. Its removal was decided according to usual rules, i.e., no air leakage and output inferior to 200 cc per day.
Results
There were 5 conversions to thoracotomy (4.2%) for a fused fissure (2 cases) and for non-controllable hemorrhage (3 cases). In 1 of these hemorrhagic complications, the planned right apicoposterior segmentectomy was finally converted into an upper lobectomy. All 5 patients had a simple postoperative course. In the 112 other patients who had a totally thoracoscopic procedure, there were 3 intraoperative complications, i.e., a partial disruption of the staple line during division of the intersegmental plane requiring endoscopic suturing. The postoperative course of these 3 patients was simple and they were discharged between postoperative day 4 and 5. Operative time ranged from 87 to 315 minutes (mean, 181±52 minutes). The estimated blood loss ranged from 0 cc (non-measurable) to 450 cc (mean, 77±81 cc). No patient needed blood transfusion. All but 12 patients had an uneventful postoperative course (90%). Complications are listed in Table 2. Out of the 12 complications, 10 were minor whereas 2 were major, i.e., requiring a reoperation. These 2 patients had an ischemia of the remaining lingula after a lingula sparing left upper lobectomy. They underwent a lingulectomy by thoracoscopy (1 patient) or by thoracotomy (1 patient), with a simple postoperative course. The drainage duration ranged from 1 to 7 days (mean, 3.3±1.9 days) and the hospital stay from 2 to 22 days (mean, 5.5±2.2 days). The final pathological results are listed in Table 3. For the 69 patients who were operated on for a suspicion of primary lung carcinoma and who had an additional lymphadenectomy, the mean number of removed hilar lymph nodes (station 10) ranged from 0 to 6 (mean, 3±2) and from station 11-12 ranged from 1 to 9 (mean, 3±2) was. The mean number of collected mediastinal lymph nodes was 21±7 and the mean number of dissected lymph node stations was 3.5±1. For patients operated on for lung cancer, the tumors were staged pathological N0 in all but 2 cases which were upstaged N1 and 4 cases which were upstaged N2.
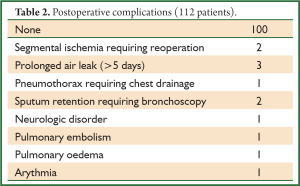
Full Table
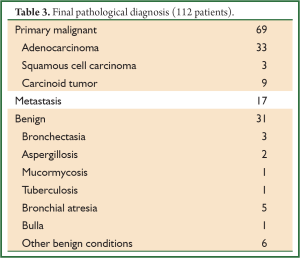
Full Table
Discussion
Anatomical landmarks
Segmentectomy is considered a challenging procedure if done by thoracotomy and even more so if it is performed thoracoscopically (2). Not only the anatomical relationships are difficult to grasp, especially for the young and less experienced surgeons, but the identification and division of the intersegmental plane is a concern. The issue is more relevant for upper segmentectomies. Not only the number of arteries arising from the pulmonary artery is variable but their distribution is sometimes difficult to appreciate because the vessels can usually not been dissected to a sufficient length. This is especially true for the ascending arteries to the right upper lobe. These arteries can supply only the posterior segment of the upper lobe or both the posterior and anterior segments. The study of preoperative computed tomography three-dimensional reconstruction helps assessing the number, size and direction of these arteries without doubt (8). Having the vascular pattern in mind helps the surgeon performing a safer dissection of the branches of the pulmonary artery, especially when the fissure is fused and/or when lymph nodes are present. In a series of 49 patients selected for VATS lobectomy, Fukuhara et al. found that preoperative three-dimensional computed pulmonary angiography was identifying the PA branches in 95% of the cases (9). In their series, only some small branches (less than 2 mm in diameter) were missed. In the beginning of our experience, most patients candidate to an upper segmentectomy had a multidetector row preoperative computed tomography (CT) angiography with three-dimensional volume-rendering reconstruction of arterial and venous anatomy. Nevertheless, CT reconstruction was not done for the lower segments since anatomical variations of the vascular supply to the lower lobes has less impact on the surgical technique and can be easily managed (8-10). As we felt more confident with the technique and the thoracoscopic vision of anatomical landmarks, the resort to preoperative CT reconstruction was progressively abandoned.
Intersegmental plane
Another difficulty faced during thoracoscopic segmentectomy is the identification and division of the intersegmental plane. When performed through a thoracotomy, this step is facilitated by the use of manual palpation which is not possible via thoracoscopy. Several methods have been described. The most common is the creation of a ventilated-deflated line by reventilating the operated lung once the segmental bronchus has been stapled. This technique has drawbacks: (I) reventilation obscures the vision and this is a much more troublesome problem than during thoracotomy; (II) the segments to be resected can be partly reventilated through the collateral canals, leading to an unclear demarcation line. Therefore some authors have suggested acting reverse, i.e., reventilating the whole lung once the segmental bronchus has been divided and then collapsing it, so that only the diseased segments remain inflated (11). Others have suggested using selected jet ventilation in the segmental bronchi to be divided (12). In emphysematous patients we have used a similar method by injecting air through the channel of a bronchofiberscope, after selective endoscopy of the segmental bronchus.
Once the intersegmental plane has been determined, the last issue is the choice of the division method. Some authors have used a combination of blunt dissection, electrocautery and application of fibrin sealant (12). When air leaks were observed, some surgeons applied mattress suture with pledgets (12). These methods have the advantage of sparing parenchyma, but comprise a risk of postoperative air leak. Actually, most authors use staplers (Table 4). Stapling is however not that easy. First, it may require using many cartridges, up to 5 in the series of Watanabe (11). Second, the limited opening of the endostaplers and the thickness of the parenchyma expose to disruption of the staples line, an adverse event that occurred twice in our series. The consequences were not serious but leaded to troublesome blood loss and required hand suturing.
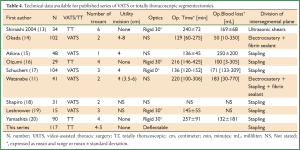
Full Table
Segmental ischemia
In our series, 2 patients had to be reoperated for an ischemia of the lingula after an upper division of the left upper lobe. In one case, it was unclear whether ischemia was related to the torsion of the remaining segment or to an injury of the lingular vein, while torsion was obvious in the second case. This complication has been reported by others (21).
Although the thoracoscopic approach offers a clear and magnified view, one of its limitations is the difficulty in obtaining a global vision of the operative field, especially as the lung is reinflated. Therefore, a wrong positioning of the remaining segment can be overlooked. In addition, securing the segment to the adjacent lobe by thoracoscopy is not that easy. When performed by thoracotomy, it is usually done by applying anchoring stiches on a partially reventilated parenchyma. This is almost impossible to perform by thoracoscopy due to the lack of space caused by reinflation of the lung. We have overcome this difficulty by applying 1 or 2 cartridges of staples, using an endostapler with no knife (Endo-TA, Covidien). Thorough examination of the remaining segment is required to avoid mispositioning. Should a reoperation be necessary, it can be performed by re-thoracoscopy (22), as occurred in one of our patient.
Lymph node dissection
Several works dealing with the issue of the validity of lymph node dissection during VATS lobectomy and segmentectomy have been recently published. Basing on a cohort of 14,473 patients, Whitson et al. have shown that survival was less after segmentectomy than after lobectomy, even for T1a tumors (23). This was confirmed by the work of Wolf et al. (23), but these authors demonstrated that survival was not statistically different between lobectomy and segmentectomy if a lymph node dissection was performed (24). Therefore, the quality of lymph node dissection during segmentectomy for lung cancer is most likely a crucial part of the procedure. Recently, Hattori et al. showed that the rate of positive lymph nodes was high for solid T1A tumors especially in case of high standardized uptake value (SUVmax). They advocate for a thorough intraoperative evaluation of lymph nodes to prevent locoregional recurrence (25). However, it seems that lobar and segmental lymph node clearance is a weak point of the thoracoscopic approach for sublobar resection. Boffa et al. have demonstrated that nodal upstaging form cN0 to pN2 was no statistically different between the open and thoracoscopic approach but that upstaging form cN0 to pN1 was significantly higher when the patient was operated on via thoracotomy (9.3% versus 6.7%) (26). This difference tended to be minimized with experience of the surgeon (26). A satisfactory clearance of stations 11 and 12 can be achieved with the use of patience, appropriate dissection and hemostatic tools and frozen section if any suspicion of nodal metastasis (24).
Tumor-free margins
In case of lung cancer, frozen section must also be used for examination of the margins after completion of segmentectomy. Indeed, local recurrence after limited resection is related not only to nodal involvement but also to the size of the lesion and to the width of the surgical margins (19).The majority of recurrences are seen when the ratio between the margin and the tumor size is less than (27). Accordingly, frozen section should be used if any doubt exists as to completeness of resection.
Conclusions
Although a totally endoscopic approach to anatomic segmentectomies can seem challenging and difficult, the operation time in our series was acceptable and the morbidity rate was low (Table 5). Combining the advantages of an endoscopic approach and an anatomic limited resection could be highly beneficial for those of the patients who fulfill the criteria of a sublobar resection. With the renewed interest for sublobar resection in the management of early stage lung carcinomas, the thoracoscopic approach may have a major role in a near future (28,29), provided the following criteria are fulfilled: (I) true anatomic resection with hilar division of bronchovascular elements; (II) adequate clearance of intersegmental lymph nodes and (III) tumor- free margins.
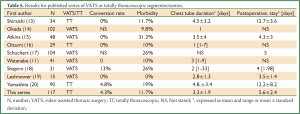
Full Table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [PubMed]
- Witte B, Wolf M, Hillebrand H, et al. Complete video-assisted thoracoscopic surgery anatomic segmentectomy for clinical stage I lung carcinoma - technique and feasibility. Interact Cardiovasc Thorac Surg 2011;13:148-52. [PubMed]
- Gossot D. eds. Atlas of endoscopic major pulmonary resections. Paris: Springer Editions, 2010.
- Shigemura N, Akashi A, Nakagiri T, et al. Complete versus assisted thoracoscopic approach: a prospective randomized trial comparing a variety of video-assisted thoracoscopic lobectomy techniques. Surg Endosc 2004;18:1492-7. [PubMed]
- Gossot D. Technical tricks to facilitate totally endoscopic major pulmonary resections. Ann Thorac Surg 2008;86:323-6. [PubMed]
- Ramos R, Girard P, Masuet C, et al. Mediastinal lymph node dissection in early-stage non-small cell lung cancer: totally thoracoscopic vs thoracotomy. Eur J Cardiothorac Surg 2012;41:1342-8; discussion 1348. [PubMed]
- Yamada S, Suga A, Inoue Y, et al. Use of multi-detector row angiography for the arrangement of video-assisted modified segmental resection. Eur J Cardiothorac Surg 2009;36:727-30. [PubMed]
- Fukuhara K, Akashi A, Nakane S, et al. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg 2008;34:875-7. [PubMed]
- Kanzaki M, Kikkawa T, Shimizu T, et al. Presurgical planning using a three-dimensional pulmonary model of the actual anatomy of patient with primary lung cancer. Thorac Cardiovasc Surg 2013;61:144-50. [PubMed]
- Watanabe A, Ohori S, Nakashima S, et al. Feasibility of video-assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775-80; discussion 780. [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [PubMed]
- Shiraishi T, Shirakusa T, Iwasaki A, et al. Video-assisted thoracoscopic surgery (VATS) segmentectomy for small peripheral lung cancer tumors: intermediate results. Surg Endosc 2004;18:1657-62. [PubMed]
- Okada M, Tsutani Y, Ikeda T, et al. Radical hybrid video-assisted thoracic segmentectomy: long-term results of minimally invasive anatomical sublobar resection for treating lung cancer. Interact Cardiovasc Thorac Surg 2012;14:5-11. [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Total thoracoscopic pulmonary segmentectomy. Eur J Cardiothorac Surg 2009;36:374-7; discussion 377. [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1.
- Shapiro M, Weiser TS, Wisnivesky JP, et al. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg 2009;137:1388-93. [PubMed]
- Leshnower BG, Miller DL, Fernandez FG, et al. Video-assisted thoracoscopic surgery segmentectomy: a safe and effective procedure. Ann Thorac Surg 2010;89:1571-6. [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [PubMed]
- Eguchi T, Kato K, Shiina T, et al. Pulmonary torsion of the lingula following a segmentectomy of the left upper division. Gen Thorac Cardiovasc Surg 2008;56:505-8. [PubMed]
- Sung HK, Kim HK, Choi YH. Re-thoracoscopic surgery for middle lobe torsion after right upper lobectomy. Eur J Cardiothorac Surg 2012;42:582-3. [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 1824-5.
- Hattori A, Suzuki K, Matsunaga T, et al. Is limited resection appropriate for radiologically “solid” tumors in small lung cancers? Ann Thorac Surg 2012;94:212-5. [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. [PubMed]
- Yang CF, D’Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [PubMed]
- Swanson SJ. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg 2010;89:S2096-7. [PubMed]


