Risk factors for continuous renal replacement therapy after surgical repair of type A aortic dissection
Introduction
Previous studies have reported a high incidence of acute kidney injury (AKI) after cardiothoracic surgery (1-3). It is associated with increased short-term mortality, morbidity, and prolonged intensive care unit stay, especially in patients who required renal replacement therapy (4,5). The incidence of AKI after aortic surgery has been reported to approximate 50% (1,6). Although mild to moderate acute kidney injuries are common, 2–15% of patients with AKI require continuous renal replacement therapy (CRRT) after aortic surgery (6-10). Despite continued progress in intensive care and renal replacement techniques in recent years, the short-term mortality remains high, ranging from 50% to over 80% in patients undergoing CRRT (5,7,11).
Type A aortic dissection (TAAD) should be managed with emergency surgical repair and is associated with a significantly increased risk of AKI (12). However, there is scarce literature on the incidence and risk factors for CRRT in patients with TAAD repair. Identification of risk factors for CRRT in patients with TAAD may lead to timely initiation of CRRT, and improve clinical outcomes. This retrospective study aims to identify the risk factors for CRRT after surgical repair in patients with TAAD.
Methods
The Ethics Committee of Beijing Anzhen Hospital approved this retrospective study (No. 2013013x).
A query of the database of Beijing Aortic Disease Center at Beijing Anzhen Hospital revealed 407 patients with TAAD surgically repaired between January 2014 and April 2015. Surgical procedures were total arch replacement and frozen elephant trunk in 330, hemiarch repair in 31, total arch replacement in 4, ascending aortic replacement in 9 and isolated composite root replacement in 20. Thirteen patients were excluded due to previous renal replacement therapy in 6, and death within 48 hours postoperatively in 7 (not attributable to renal causes). As a result, 330 patients with total arch replacement and frozen elephant trunk procedure were included in this study.
The surgical techniques of total arch replacement and frozen elephant trunk (i.e., the Sun operation) have been described in detail (13). Briefly, it is performed under deep hypothermic circulatory arrest (DHCA) and antegrade selective cerebral perfusion. An open stented graft, Cronus (MicroPort Medical, Shanghai, China), which differs from the E-vita and Thoraflex grafts (14), is deployed into the descending aorta and total arch replacement is done with a 4-branched vascular graft. Distal reperfusion is begun once the descending aortic anastomosis is completed, and the left carotid artery is reconstructed first (after which rewarming is started and the brain is perfused bilaterally), followed by the ascending aorta (to resume myocardial perfusion), then the left subclavian artery, and finally, the innominate artery. Concomitant procedures included composite graft root replacement in 138 (41.8%) and ascending aortic replacement in 188 patients (57.0%).
The endpoint of this study was the need for CRRT after surgical repair of TAAD. The criteria for postoperative CRRT are shown in Table 1 (15). Variables considered in the univariate analysis included gender, age, body mass index (BMI), hypertension, diabetes, smoking, history of cardiac surgery, preoperative serum creatinine (sCr), left ventricular ejection fraction (LVEF), emergency operation, D-dimmer, the times of cardiopulmonary bypass (CPB), aortic cross-clamp and DHCA, the amount of red blood cell and frozen fresh plasma transfused intraoperatively, and reexploration for bleeding.

Full table
Statistical analyses
Data were analyzed with SPSS for Windows version 20.0 (SPSS IBM Corp., Armonk, NY, USA). Variables were expressed as mean ± standard deviation or percentages, and compared between patients with and without CRRT using the Student t test or Pearson χ2 test as appropriate. Univariate analysis was used to evaluate variables that may be associated with CRRT. Variables with a P value <0.05 in univariate analysis were assessed with multivariate analysis using a forward stepwise binary logistic regression model. All statistical tests were 2-sided and any P value of <0.05 was considered statistically significant.
Results
The mean age of patients was 47.1±10.2 years for the whole cohort (range, 18–73 years) and 242 (73.3%) were male. Previous cardiac surgery was present in 20 patients (6.1%). There were 248 (75.2%) emergency operations. Reexploration for bleeding was required in 13 patients (3.9%).
CRRT was required in 38 patients (11.5%). Mean age was 50.7±10.0 years (range, 27–71 years) and there were 27 men (71.1%). Operative death occurred in 12 patients (3.6%, 12/330). The mortality rate was 23.7% (9/38) in patients with CRRT and 1.0% (3/292) in those without CRRT (P<0.001).
As shown in Table 2, univariate analysis found that nine variables were associated with postoperative CRRT: age (50.7±10.0 vs. 46.7±10.2, P=0.023), sCr (135.0±154.2 vs. 85.7±37.0 µmol/L, P<0.001), emergency operation (89.5% vs. 73.3%, P=0.030), CPB time (265.2±98.8 vs. 199.7±44.2 minutes, P<0.001), cross-clamp time (144.6±54.8 vs. 116.3±33.2 minutes, P<0.001), the amount of red blood cell (8.0±5.2 vs. 3.7±3.3 unit, P<0.001) and fresh frozen plasma (507.8±350.3 vs. 784.2±488.5 mL, P<0.001) transfused intraoperatively, preoperative D-dimmer level (11,361.0 vs. 2,856.7 mg/L, P<0.001) and reexploration for bleeding (15.8% vs. 2.4%, P<0.001).
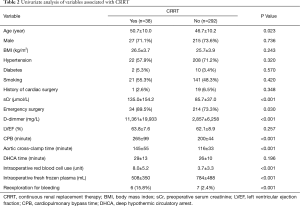
Full table
In multivariate analysis, risk factors for CRRT were CPB time (minute) [odds ratio (OR), 1.018; 95% confidence interval (CI), 1.007–1.029; P=0.002], preoperative sCr level (µmol/L) (OR, 1.008; 95% CI, 1.000–1.015; P=0.040), and the amount of red blood cell transfused intraoperatively (unit) (OR, 1.206; 95% CI, 1.077–1.350; P=0.001) (Table 3).
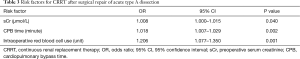
Full table
Discussion
In this retrospective study, the incidence of CRRT after surgical repair of TAAD was 11.5%, which is comparable to that reported in previous studies (8,9,16). Roh and colleagues (9) reported that the incidence of renal replacement therapy was 11% in 98 patients undergoing surgical repair of acute dissection involving the ascending aorta (67%), aortic arch (41%), descending aorta (41%), and aortic valve (5%). Arnaoutakis and associates (8) reported 8% of patients (22/267) required renal replacement therapy after aortic arch surgery. Recently Motomura et al. (16) reported that the incidence of acute renal failure requiring dialysis was 7% in 4,707 patients undergoing thoracic aortic surgery, which involved the aortic root in 10%, ascending aorta in 47%, aortic arch in 44%, distal arch in 21%, descending aorta in 27% and thoracoabdominal aorta in 8%.
Similar to previous reports (8,11,12), sCr level was also identified as a risk factor for CRRT in this study, which shows a significant influence of elevations in the preoperative creatinine serum levels on postoperative renal function. Hypotension, nephrotoxins and inflammation may lead to acute renal failure, followed by a substantial increase (>50%) in serum creatinine (17). Hypotension may result from cardiac tamponade, dehydration or the involvement of renal artery by the dissection process. Reduction of renal blood flow, whether generalized or localized, plays a critical role in the occurrence of AKI (18). Ren et al. (19) reported bilateral renal artery involvement were predictors for preoperative AKI. Nephrotoxins include drugs and contrasts. Inflammatory response plays a major role in the development of acute aortic dissection and may contribute to the occurrence of AKI. Elevation of many inflammatory markers is associated with worse prognosis (20-22). Therapeutic strategies aimed at reducing kidney damage and accelerating function recovery contain nonpharmacologic, pharmacologic, and dialytic approaches (23). Therefore, any therapy that can treat hypotension, nephrotoxins and inflammation may be helpful improve the outcome, such as fluid, colloid administration for hemodynamic optimization. Elahi et al. (24) suggested that early initiation of CRRT may reduce mortality and mortality in patients with severe AKI after cardiac surgery.
Besides the serum creatinine level, the CPB time (minute) was identified as another risk factor for CRRT. CPB is associated with elevations in levels of systemic inflammatory factors and activation of inflammatory response, which may lead to renal injury (23). Ho and colleagues (25) found that all patients under CPB experienced the “initiation phase” of ischemia-reperfusion kidney injury, which was characterized by renal artery vasoconstriction and increased tubular oxygen consumption, leading to impaired renal oxygenation and proximal tubular dysfunction (26). With longer duration of CPB, more severe hypotensive periods may occur frequently. This entails prolonged period in which the mean arterial pressure is below the optimal renal autoregulation threshold (27), which may aggravate the inflammatory response and induce ischemic kidney damage (18,28,29). In addition, CPB is associated some degrees of hemolysis and release of free hemoglobin, which act as an endogenous toxin through the release of iron, especially in the presence of low ferritin level (30). Whether patients recover without sequela or develop AKI by progressing into the “extension phase” of kidney injury largely depends on the severity of the ensuing inflammatory response, renal hypoxia, and oxidative stress (25).
Another risk factor for CRRT is the units of red blood cell transfused during operation, which has not been reported previously. Roh and colleagues (9) identified increased transfusion of red blood cell in the intensive care unit as a risk factor for CRRT. The quality of the red blood cells can decline with storage, leading to morphologic changes that impair tissue oxygen delivery. Transfused red cells may be unable to properly load and unload oxygen and have negative effects on renal function (31). The erythrocyte membrane undergoes changes that are mostly irreversible and becomes less deformable and more fragile during storage. Within an hour of transfusion, up to 30% of the transfused erythrocytes are either hemolysed, potentially leading to the presence of free hemoglobin in the circulation, or removed from the circulation by macrophages (32). By promoting a proinflammatory state, impairing tissue oxygen delivery, and exacerbating tissue oxidative stress, red blood cell transfusion can be an important instigator of kidney injury. This risk is likely to be influenced by the number of units transfused (33). By some estimates, transfusion of 2 units of red blood cells could increase the plasma free hemoglobin by 10-fold above normal levels (34). The hemolysis leads to an increase in the circulating catalytic iron (31). Free hemoglobin can cause microcirculatory dysfunction through nitric oxide scavenging and free iron is a potent pro-oxidant (34,35), both highly toxic to the kidney and other organs. Therefore, improvement in blood conservation techniques may help reduce the incidence of CRRT after surgical repair of type A aortic dissection.
Although Chertow and associates (4) reported that renal failure requiring renal replacement therapy after cardiac surgery is a risk factor for mortality, there is a trend towards early initiation of renal replacement therapy, which is believed to be more beneficial than late initiation (24). In the present cohort, patients with CRRT had a 23-fold higher mortality than those without. Such high mortality of patients with CRRT implies that starting CRRT earlier may improve clinical outcomes by alleviating kidney damage and promoting function recovery.
This retrospective study has several limitations. Most important is its relatively small sample size, which affects the power of statistical analysis, especially in identification of risk factors. Another concern pertains to the young average age of patients in this study (47.1±10.2 years). We did not use eGFR as a standard because eGFR is affected by ethnicity and gender and calculated by different methods in different labs. In addition, all patients had a frozen elephant trunk and total arch replacement procedure in this series, while other centers may not have such a higher percentage. Therefore, the results of this study may not be directly applicable to other centers. Despite these limitations, identification of risk factors for CRRT in patients with TAAD may help in initiating CRRT timely, adjusting management goals and predicting prognosis in clinical practice. It would be reasonable to expect, that any intervention which can reduce perioperative transfusion or control CPB time may be protective against AKI.
Conclusions
In this series of patients with TAAD, preoperative serum creatinine (µmol/L), CPB time (minute) and the amount of red blood cell transfused intraoperatively (unit) were risk factors for CRRT after surgical repair.
Acknowledgements
Funding: This study was supported in part by the National Key Technologies Research and Development Program (grant 2015BA112B03) and Special Research Fund for Public Health and Welfare (grant 201402009).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committee of Beijing Anzhen Hospital approved this retrospective study (No. 2013013x) and written informed consent was obtained from all patients.
References
- Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119:2444-53. [Crossref] [PubMed]
- Kuitunen A, Vento A, Suojaranta-Ylinen R, et al. Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg 2006;81:542-6. [Crossref] [PubMed]
- Kim MY, Jang HR, Huh W, et al. Incidence, risk factors, and prediction of acute kidney injury after off-pump coronary artery bypass grafting. Ren Fail 2011;33:316-22. [Crossref] [PubMed]
- Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998;104:343-8. [Crossref] [PubMed]
- Englberger L, Suri RM, Greason KL, et al. Deep hypothermic circulatory arrest is not a risk factor for acute kidney injury in thoracic aortic surgery. J Thorac Cardiovasc Surg 2011;141:552-8. [Crossref] [PubMed]
- D'Onofrio A, Cruz D, Bolgan I, et al. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail 2010;16 Suppl 1:S32-6. [Crossref] [PubMed]
- Kowalik MM, Lango R, Klajbor K, et al. Incidence- and mortality-related risk factors of acute kidney injury requiring hemofiltration treatment in patients undergoing cardiac surgery: a single-center 6-year experience. J Cardiothorac Vasc Anesth 2011;25:619-24. [Crossref] [PubMed]
- Arnaoutakis GJ, Bihorac A, Martin TD, et al. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg 2007;134:1554-60; discussion 1560-1. [Crossref] [PubMed]
- Roh GU, Lee JW, Nam SB, et al. Incidence and risk factors of acute kidney injury after thoracic aortic surgery for acute dissection. Ann Thorac Surg 2012;94:766-71. [Crossref] [PubMed]
- Di Bartolomeo R, Di Marco L, Armaro A, et al. Treatment of complex disease of the thoracic aorta: the frozen elephant trunk technique with the E-vita open prosthesis. Eur J Cardiothorac Surg 2009;35:671-5; discussion 675-6. [Crossref] [PubMed]
- Bove T, Calabrò MG, Landoni G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth 2004;18:442-5. [Crossref] [PubMed]
- Geirsson A, Szeto WY, Pochettino A, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg 2007;32:255-62. [Crossref] [PubMed]
- Ma WG, Zheng J, Liu YM, et al. Dr. Sun's procedure for type A aortic dissection: total arch replacement using tetrafurcate graft with stented elephant trunk implantation. Aorta (Stamford) 2013;1:59-64. [Crossref] [PubMed]
- Ma WG, Zheng J, Sun LZ, et al. Open stented grafts for frozen elephant trunk technique: technical aspects and current outcomes. Aorta (Stamford) 2015;3:122-35. [Crossref] [PubMed]
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 2012;380:756-66. [Crossref] [PubMed]
- Motomura N, Miyata H, Tsukihara H, et al. Risk model of thoracic aortic surgery in 4707 cases from a nationwide single-race population through a web-based data entry system: the first report of 30-day and 30-day operative outcome risk models for thoracic aortic surgery. Circulation 2008;118:S153-9. [Crossref] [PubMed]
- Brown JR, Cochran RP, Dacey LJ, et al. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation 2006;114:I409-13. [Crossref] [PubMed]
- Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 2011;121:4210-21. [Crossref] [PubMed]
- Ren HM, Wang X, Hu CY, et al. Relationship between acute kidney injury before thoracic endovascular aneurysm repair and in-hospital outcomes in patients with type B acute aortic dissection. J Geriatr Cardiol 2015;12:232-8. [PubMed]
- Luo F, Zhou XL, Li JJ, et al. Inflammatory response is associated with aortic dissection. Ageing Res Rev 2009;8:31-5. [Crossref] [PubMed]
- Kuehl H, Eggebrecht H, Boes T, et al. Detection of inflammation in patients with acute aortic syndrome: comparison of FDG-PET/CT imaging and serological markers of inflammation. Heart 2008;94:1472-7. [Crossref] [PubMed]
- Shimada S, Nakamura H, Kurooka A, et al. Fever associated with acute aortic dissection. Circ J 2007;71:766-71. [Crossref] [PubMed]
- Bellomo R, Auriemma S, Fabbri A, et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI). Int J Artif Organs 2008;31:166-78. [PubMed]
- Elahi M, Asopa S, Pflueger A, et al. Acute kidney injury following cardiac surgery: impact of early versus late haemofiltration on morbidity and mortality. Eur J Cardiothorac Surg 2009;35:854-63. [Crossref] [PubMed]
- Ho J, Lucy M, Krokhin O, et al. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am J Kidney Dis 2009;53:584-95. [Crossref] [PubMed]
- Redfors B, Bragadottir G, Sellgren J, et al. Dopamine increases renal oxygenation: a clinical study in post-cardiac surgery patients. Acta Anaesthesiol Scand 2010;54:183-90. [Crossref] [PubMed]
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008;3:1266-73. [Crossref] [PubMed]
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006;1:19-32. [Crossref] [PubMed]
- Flemming B, Seeliger E, Wronski T, et al. Oxygen and renal hemodynamics in the conscious rat. J Am Soc Nephrol 2000;11:18-24. [PubMed]
- Davis CL, Kausz AT, Zager RA, et al. Acute renal failure after cardiopulmonary bypass in related to decreased serum ferritin levels. J Am Soc Nephrol 1999;10:2396-402. [PubMed]
- de Vries B, Walter SJ, von Bonsdorff L, et al. Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation 2004;77:669-75. [Crossref] [PubMed]
- Lasocki S, Longrois D, Montravers P, et al. Hepcidin and anemia of the critically ill patient: bench to bedside. Anesthesiology 2011;114:688-94. [Crossref] [PubMed]
- Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth 2012;109 Suppl 1:i29-i38. [Crossref] [PubMed]
- Vermeulen Windsant IC, Hanssen SJ, Buurman WA, et al. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg 2011;142:1-11. [Crossref] [PubMed]
- Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 2011;124:465-76. [Crossref] [PubMed]


