Occupational exposure and lung cancer
Lung cancer is estimated to be the leading cause of cancer death worldwide during the last decades. By 2008, there were an approximately 1.6 million of new lung cancer cases (12.7% of all new diagnosis for cancer) while it was the most common cause of cancer death (≈1.4 million deaths, 18.2% of the total) (1). Most cases (56% of total) and higher death rates (538/100,000 vs. 410/100,000) are present in less developed countries. A recent study from Europe showed that even though it was the fourth most common cancer-below female breast cancer, colorectal and prostate—it was the leading cause of cancer death (353,000 deaths for 2012) (2). In the USA, lung cancer is the second most common cancer for both sexes but the leading cause of cancer death for both men and women (3). It is worth mention that only recently a trend across decreasing incidence and mortality rates among US women was detected for the first time (4).
Carcinogenesis is a complex, multifactorial process in which genetic (5,6) as well as and environmental causative factors play an interrelated role that lead to uncontrolled cell growth. Cigarette smoking is considered the leading cause of lung cancer, as it is the main causative agent for about 80% to 90% of cases in countries where the prevalence of cigarette smoking is high (7). Changes of smoking habits in populous, developing countries like China will alter the world map of lung cancer (8).
However it is estimated that about 10-20% of lung cancer cases are detected among never smokers with great geographic variability (9,10). Approximately 300,000 deaths/year due to lung cancer worldwide could not be attributed to cigarette smoking (11). If we categorize lung cancer among never smokers as a separate group we will find that it is the seventh most common cause of cancer death, well above cervix, pancreas or prostate cancer (12). Many etiologic factors of lung cancer—other than cigarette smoking—have been identified: exposure to environmental cigarette smoke (passive smoking) (13); occupational exposure to agents like asbestos and hard metals (14); exposure to radiation, especially radon (15,16); and exposure to indoor and outdoor air pollution (17,18).
Lung cancer, leukemia, and mesothelioma are the most common forms of occupational cancer (19). Lung cancer is considered to be the most common among occupational related cancers (20). The precise percentage of patients with lung cancer who had been exposed to occupational carcinogens that contributed to the development of the disease is difficult to be estimated due to a wide range in the intensity of exposure, different genetic/ethnicity background and smoking history. However a figure of approximatelly 10% is referred by some authors (21). Occupational exposure to agents that are associated with lung cancer development is very important as: (I) sometimes physicians do not take detailed occupational history in patients with lung cancer; (II) tobacco smoke has synergic effect with many occupational carcinogens (22,23) and (III) patients with lung cancer after sufficient exposure to an agent which is definitely associated with the disease have the right for financial compensation. On the contrary are often underreported in everyday clinical practice (24).
The International Agency for Research on Cancer (IARC)—an independent scientific section of the World Health Organization—has divided chemical/occupational/environmental/physical and biological agents into 4 categories according to their carcinogenetic potential (Table 1) (25). We should mention that in the term “agent” are also included some behavioral or cultural aspects. It is obvious that the above division is a dynamic process which evolves parallel with the current scientific literature (agents of Group 2A may be upgraded to Group 1 in the future). Table 2 presents all carcinogenic agents which are causally related with lung cancer according to the last classification by IARC (26).

Full Table
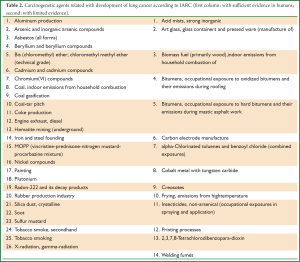
Full Table
Occupational agents/activities that are associated with increased risk for lung cancer are:
- Mining and usage of asbestos in industry or manufacture (asbestos cement products, thermal and electrical insulation in construction and shipyard work, brakes, textile industry) (27,28). It seems that asbestos fibers size (long and thin) is a strong predictor of lung cancer mortality (29). Even though there is still a controversy in the literature, probably chrysotile is considered less carcinogenetic than amphibole forms of asbestos (27,30);
- Usage of arsenic and arsenic compounds (antifungal outdoor wood preservatives, agricultural industry of pesticides, herbicides and insecticides, manufacture of non-ferrous alloys, glass-manufacturing, electronics industry) (31,32);
- Exposure to beryllium and beryllium oxide (nuclear technology, X-ray and radiation technology, dental applications and as beryllium-copper alloys in the electronics, aerospace technology, automotive) (33-35);
- Exposure to bis (chloromethyl) ether and chloromethyl methyl ether (36,37). Nowadays the possibility for exposure is low because their uses is strictly regulated, are no longer produced in large quantities and almost always are used in closed containers for the synthesis of other chemicals. They are used as a reagent in the manufacture of plastics, ion-exchange resins and polymers;
- Industrial use of cadmium (38,39) [nickel-cadmium (Ni-Cd) batteries is its major use, pigments, coatings and plating in the form of cadmium-alloys, stabilizers for plastics];
- Exposure to substances as a painter (40-42). Paint is a complex substance that is composed of pigment particles (titanium dioxide, micro-crystalline carbon and azo pigments which are based on aromatic amines), a binder which is usually a resin or a drying oil, a volatile solvent or water and additives in small quantities that give special properties to paints or coatings. Painters are exposed to the chemicals during their application (mainly solvents) and removal (pigments, resins, silica);
- Nickel-producing industries (mining, milling, smelting, and refining) as well as nickel-using industries (alloys and stainless steel manufacture is its major use, electroplating, welding, grinding and cutting) (43-45). Workers in the former industries are exposed to insoluble nickel whereas soluble nickel is the predominant exposure in the later;
- Exposure to chromium (VI) which occurs during production, use and welding of chromium-containing metals and alloys (manufacture of fabricated metal products, machinery and transport equipment); electroplating; production and use of chromium-containing compounds (pigments, paints, catalysts, chromic acid, tanning agents, and pesticides) (46);
- Exposure to silica dust and its crystalline form (quartz) (47,48). The three main commercial silica product categories are: sand and gravel (manufacture of glass, ceramics, foundry and abrasive activities), quartz crystals (jewellery, electronics and optical components industries) and diatomites (paint and paper industry, synthetic rubber goods, scourer in polishes and cleaners). Also workers in mines and quarries, constructions, crushed stone industries and sandblasting are severely exposed. The presence of silicosis increase further the risk for lung cancer (49);
- Workers in aluminium production who are primarily exposed to polycyclic aromatic hydrocarbons and also to sulfur dioxide and fluorides, various aluminium compounds, chromium and nickel. The risk for lung cancer seems to be increased but studies are still controversial (50-52);
- Coke-ovens workers (coke production) are mainly exposed to polycyclic aromatic hydrocarbons. Increased risk for lung cancer has been proved by some but not all studies (53,54);
- Workers in the rubber-manufacturing industry are exposed to dusts and fumes as well as N-nitrosamines, polycyclic aromatic hydrocarbons, solvents and phthalates. There is sufficient evidence for excess lung cancer incidence and mortality (42,55-57);
- Recently a Working Group of IARC concluded that diesel exhaust is a cause of lung cancer (58) but other authors believe that scientific data from occupational studies is not enough to support the above hypothesis (59);
- Second-hand tobacco smoke (passive-smoking) represents an occupational exposure for workers in bars, restaurants, public buildings and educational institutions especially in countries without smoke free legislations in public places (60,61);
- There is some evidence that workers in the nuclear industry demonstrate an increased risk for lung cancer mortality (62).
As a general rule we could assume that for most carcinogenic agents it has been estimated a dose-response relationship between cumulative exposure and the risk for lung cancer. Also there is usually a lag period that ranges 10-30 years from initial exposure to the time point that relative risk increases to statistical significance. Occupational studies investigating the role of a potential carcinogenic agent on lung cancer incidence or mortality is extremely difficult to come to a definite conclusion due to the presence of various confounders (e.g., cigarette smoking, socioeconomic conditions, diet, air pollution, ethnical differences, simultaneous exposure to several carcinogenetic agents). In patients with lung cancer—especially among never smokers or those with unremarkable smoking history—taking a detailed occupational history (jobs and their duration, the precise workplace and the exact activity, presence of fumes/gases/dusts, use of protective measures) is fundamental but many times physicians underreported it. National Work Health Policy should guarantee a comprehensive plan of occupational hygiene (protection, follow up of air concentration for dangerous agents, regular medical examinations) especially for developing countries with industry expansion.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [PubMed]
- Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on thestatus of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008;100:1672-94. [PubMed]
- Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst 2011;103:714-36. [PubMed]
- Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res 2000;77:81-137. [PubMed]
- Gazdar A, Franklin WA, Brambilla E, et al. Genetic and molecular alterations. In: Travis WD, Brambilla E, Müller-Hermelink HK, et al. eds. Pathology and genetics: tumours of the lung, pleura, thymus and heart. Lyon: IARC Press, 2004.
- Peto R, Lopez AD, Boreham J, et al. eds. Mortality from smoking in developed countries 1950-2000. Indirect estimates from national vital statistics. New York: Oxford University Press, 1994.
- Yang L, Parkin DM, Ferlay J, et al. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev 2005;14:243-50. [PubMed]
- Toh CK, Gao F, Lim WT, et al. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J Clin Oncol 2006;24:2245-51. [PubMed]
- Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 2008;5:e185. [PubMed]
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer 2007;7:778-90. [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General, US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health Atlanta, GA 2006.
- Alberg AJ, Yung RC, Strickland P, et al. Respiratory cancer and exposure to arsenic, chromium, nickel and polycyclic aromatic hydrocarbons. Clin Occup Environ Med 2002;2:779-801.
- Lubin JH, Boice JD Jr, Edling C, et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Natl Cancer Inst 1995;87:817-27. [PubMed]
- US Environmental Protection Agency. Technical support document for the 1992 citizen’s guide to Radon, Washington, 1992.
- Hosgood HD 3rd, Boffetta P, Greenland S, et al. In-home coal and wood use and lung cancer risk: a pooled analysis of the International Lung Cancer Consortium. Environ Health Perspect 2010;118:1743-7. [PubMed]
- Turner MC, Krewski D, Pope CA 3rd, et al. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med 2011;184:1374-81. [PubMed]
- Driscoll T, Nelson DI, Steenland K, et al. The global burden of disease due to occupational carcinogens. Am J Ind Med 2005;48:419-31. [PubMed]
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 1981;66:1191-308. [PubMed]
- De Matteis S, Consonni D, Bertazzi PA. Exposure to occupational carcinogens and lung cancer risk. Evolution of epidemiological estimates of attributable fraction. Acta Biomed 2008;79:34-42. [PubMed]
- Frost G, Darnton A, Harding AH. The effect of smoking on the risk of lung cancer mortality for asbestos workers in Great Britain (1971-2005). Ann Occup Hyg 2011;55:239-47. [PubMed]
- Saracci R. The interactions of tobacco smoking and other agents in cancer etiology. Epidemiol Rev 1987;9:175-93. [PubMed]
- Rushton L, Hutchings S, Brown T. The burden of cancer at work: estimation as the first step to prevention. Occup Environ Med 2008;65:789-800. [PubMed]
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans, Lyon, 2006.
- International Agency for Research on Cancer (Available online: ). eds. Agents classified by the IARC monographs, Volumes 1-107. Lyon: IARC, 2013.
- Hodgson JT, Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg 2000;44:565-601. [PubMed]
- Berman DW, Crump KS. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit Rev Toxicol 2008;38:49-73. [PubMed]
- Stayner L, Kuempel E, Gilbert S, et al. An epidemiological study of the role of chrysotile asbestos fibre dimensions in determining respiratory disease risk in exposed workers. Occup Environ Med 2008;65:613-9. [PubMed]
- Stayner LT, Dankovic DA, Lemen RA. Occupational exposure to chrysotile asbestos and cancer risk: a review of the amphibole hypothesis. Am J Public Health 1996;86:179-86. [PubMed]
- Enterline PE, Henderson VL, Marsh GM. Exposure to arsenic and respiratory cancer. A reanalysis. Am J Epidemiol 1987;125:929-38. [PubMed]
- Lubin JH, Moore LE, Fraumeni JF Jr, et al. Respiratory cancer and inhaled inorganic arsenic in copper smelters workers: a linear relationship with cumulative exposure that increases with concentration. Environ Health Perspect 2008;116:1661-5. [PubMed]
- Steenland K, Ward E. Lung cancer incidence among patients with beryllium disease: a cohort mortality study. J Natl Cancer Inst 1991;83:1380-5. [PubMed]
- Ward E, Okun A, Ruder A, et al. A mortality study of workers at seven beryllium processing plants. Am J Ind Med 1992;22:885-904. [PubMed]
- Schubauer-Berigan MK, Deddens JA, Steenland K, et al. Adjustment for temporal confounders in a reanalysis of a case-control study of beryllium and lung cancer. Occup Environ Med 2008;65:379-83. [PubMed]
- Gowers DS, DeFonso LR, Schaffer P, et al. Incidence of respiratory cancer among workers exposed to chloromethyl-ethers. Am J Epidemiol 1993;137:31-42. [PubMed]
- Weiss W, Nash D. An epidemic of lung cancer due to chloromethyl ethers. 30 years of observation. J Occup Environ Med 1997;39:1003-9. [PubMed]
- Sorahan T, Lancashire RJ. Lung cancer mortality in a cohort of workers employed at a cadmium recovery plant in the United States: an analysis with detailed job histories. Occup Environ Med 1997;54:194-201. [PubMed]
- Nawrot T, Plusquin M, Hogervorst J, et al. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol 2006;7:119-26. [PubMed]
- Guha N, Merletti F, Steenland NK, et al. Lung cancer risk in painters: a meta-analysis. Environ Health Perspect 2010;118:303-12. [PubMed]
- Bachand A, Mundt KA, Mundt DJ, et al. Meta-analyses of occupational exposure as a painter and lung and bladder cancer morbidity and mortality 1950-2008. Crit Rev Toxicol 2010;40:101-25. [PubMed]
- Pronk A, Coble J, Ji BT, et al. Occupational risk of lung cancer among lifetime non-smoking women in Shanghai, China. Occup Environ Med 2009;66:672-8. [PubMed]
- Grimsrud TK, Berge SR, Haldorsen T, et al. Exposure to different forms of nickel and risk of lung cancer. Am J Epidemiol 2002;156:1123-32. [PubMed]
- Sorahan T, Williams SP. Mortality of workers at a nickel carbonyl refinery, 1958-2000. Occup Environ Med 2005;62:80-5. [PubMed]
- Sivulka DJ, Seilkop SK. Reconstruction of historical exposures in the US nickel alloy industry and the implications for carcinogenic hazard and risk assessments. Regul Toxicol Pharmacol 2009;53:174-85. [PubMed]
- Cole P, Rodu B. Epidemiologic studies of chrome and cancer mortality: a series of meta-analyses. Regul Toxicol Pharmacol 2005;43:225-31. [PubMed]
- Lacasse Y, Martin S, Gagné D, et al. Dose-response meta-analysis of silica and lung cancer. Cancer Causes Control 2009;20:925-33. [PubMed]
- Pelucchi C, Pira E, Piolatto G, et al. Occupational silica exposure and lung cancer risk: a review of epidemiological studies 1996-2005. Ann Oncol 2006;17:1039-50. [PubMed]
- Lacasse Y, Martin S, Simard S, et al. Meta-analysis of silicosis and lung cancer. Scand J Work Environ Health 2005;31:450-8. [PubMed]
- Armstrong BG, Gibbs G. Exposure-response relationship between lung cancer and polycyclic aromatic hydrocarbons (PAHs). Occup Environ Med 2009;66:740-6. [PubMed]
- Friesen MC, Demers PA, Spinelli JJ, et al. Comparison of two indices of exposure to polycyclic aromatic hydrocarbons in a retrospective aluminium smelter cohort. Occup Environ Med 2007;64:273-8. [PubMed]
- Romundstad P, Andersen A, Haldorsen T. Cancer incidence among workers in six Norwegian aluminum plants. Scand J Work Environ Health 2000;26:461-9. [PubMed]
- Costantino JP, Redmond CK, Bearden A. Occupationally related cancer risk among coke oven workers: 30 years of follow-up. J Occup Environ Med 1995;37:597-604. [PubMed]
- Hurley JF, Archibald RM, Collings PL, et al. The mortality of coke workers in Britain. Am J Ind Med 1983;4:691-704. [PubMed]
- Szymczak W, Sobala W, Wilczyńska U, et al. Assessment of risk of death due to malignant neoplasms induced by occupational exposure in a rubber footwear plant. Med Pr 2003;54:221-8. [PubMed]
- Kogevinas M, Sala M, Boffetta P, et al. Cancer risk in the rubber industry: a review of the recent epidemiological evidence. Occup Environ Med 1998;55:1-12. [PubMed]
- Mundt KA, Weiland SK, Bucher AM, et al. An occupational cohort mortality study of women in the German rubber industry: 1976 to 1991. J Occup Environ Med 1999;41:807-12. [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chemical agents and related occupations. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt F):9-562.
- Gamble JF, Nicolich MJ, Boffetta P. Lung cancer and diesel exhaust: an updated critical review of the occupational epidemiology literature. Crit Rev Toxicol 2012;42:549-98. [PubMed]
- Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol 2007;36:1048-59. [PubMed]
- Veglia F, Vineis P, Overvad K, et al. Occupational exposures, environmental tobacco smoke, and lung cancer. Epidemiology 2007;18:769-75. [PubMed]
- Cardis E, Vrijheid M, Blettner M, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat Res 2007;167:396-416. [PubMed]


