Use of multidetector-row computed tomography scan to detect pannus formation in prosthetic mechanical aortic valves
Aortic valve replacement (AVR) is the second most common cardiothoracic procedure performed in the United States (1) and Europe (2). Surgically or percutaneously implanted, the results of AVRs are uniformly good and the number of patients have been steadily increasing over the last decades (3). Although most of the newly implanted devices are tissue valves, yet a sizable number are mechanical especially in young patients with rheumatic disease. The latter group is predominant in non-Caucasian populations where the rheumatic disease is the most common aetiology (4).
In parallel, during the same years computed tomography (CT) scan technology evolved substantially offering excellent resolution and the ability to identify the commonest and most challenging complications of AVR (5). In particular, ECG-gated CT scan proved to be more reliable than echocardiography in identifying pannus, the most insidious long-term complication of mechanical valves.
CT scan technique
Images are usually acquired using retrospective ECG-gated protocols with cardiac phase reconstruction at 5% to 10% RR interval increments. Both systolic (at 20% to 40% of RR interval) and diastolic phase (at 70% to 90% RR interval) are usually necessary. With these modalities, more than 90% of the valves can be accurately evaluated (6). As opposed to retrospective triggering, prospective ECG-gated protocols tend to generate less artifacts and require lesser radiation doses. However, they can be used only in patients with regular heart rate. When the patients are in atrial fibrillation, short acting beta-blockers can be used during the acquisition phase. However, with faster heart rates best results are obtained with dual source CT scan.
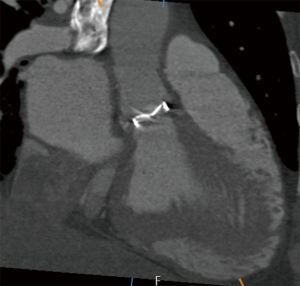
CT data are reconstructed by a separate workstation for post-processing where oblique coronal or oblique sagittal images of the aortic root are assembled (Figure 1). By using a multiplanar image technique, profile and enface views of the prosthetic aortic valve showing the least amount of motion artifacts are generated. The presence or absence of a subprosthetic pannus is determined by the examiner as the appearance of a low-attenuation lesion or a calcified lesion protruded into the valvular strut, beneath the prosthetic valve. The degree of attenuation for the pannus has been consistently found greater than 145 Hounsfield Units (HU). Great care is necessary to exclude pseudolesions resulting from beam-hardening artifacts. These images usually do not persist through the cardiac cycle and appear as ill-defined lesions projecting from the prosthesis and have lower attenuation than the myocardium. Finally, the circular angle of extension of the subvalvular pannus is evaluated. The geometric orifice area (GOA) of each prosthetic valve is measured and the ratio of encroachment of subprosthetic pannus derived. The effective orifice area (EOA) of the prosthetic valve is measured by subtracting the area of the pannus from the GOA. The encroachment ratio (as a percentage) is defined as: [(GOA − EOA)/GOA ×100%] (7).
Pannus, thrombus or mismatch
In order to understand the basic principles underlying the recognition of pannus on CT scans it can be useful to recall some of the characteristics of this structure.
Histologic examination of pannus (8) from explanted valves shows that it comprises three different layers. A lumen layer consisting of endothelial cells, an internal medial layer of spirally oriented spindle cells identifiable as myofibroblasts and an external medial layer of collagen and fibrous tissue. Chronic inflammatory cells infiltration can be observed in the inner medial layer. Additionally, at the level of native annuli contact with the prosthetic sewing cuff a “stump” lesion can be observed showing a chronic foreign body reaction with giant cells and macrophages infiltration.
This organized structure which includes a fibrous layer, demonstrates a high attenuation capacity at CT scan with values exceeding 145 HU (9).
In contrast, thrombi are mainly composed by non-nucleated red cells packed in a platelets/fibrin matrix. An acute thrombus has a CT attenuation value that is dependent largely on the proportions of red blood cells. It is therefore of no surprise that the HU value of acute thrombus is similar to published attenuation measurements of whole blood.
In organized thrombi, endothelial cells sprout in irregular clefts in a few days and, depending on conditions, cellular penetration can advance approximately of 1 mm per day. Fibroblastic proliferation occurs along with capillary formation. Collagen may be detected after in 5–10 days (10). However, organized thrombi show little fibrinogen content as compared to pannus.
The clot contraction owing to an increase in haemoglobin and iron concentration, occurs after capillary formation and the proliferation of fibroblasts. As a consequence of this process, the attenuation of chronic thrombus is generally higher, ranging between 90 and 145 HU (11,12).
Frequently, pannus and thrombus coexist. The slow accretion of pannus can generate areas of flow stagnation and trigger the formation of a thrombus. Vitale over 56 cases of pannus leading to re-operation could find an associated thrombosis in 36 (64%) patients (13).
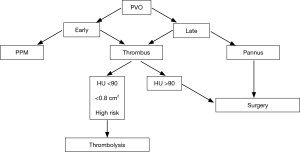
At CT scan it is important to differentiate recent (fresh) from chronic (organized) thrombosis as the former is usually suitable for thrombolytic treatment which is the case when the attenuation is <90 HU (11-14) (Figure 2).
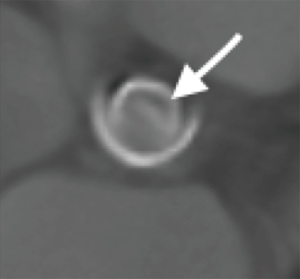
In fact, Ozkan (14) reported 85.5% success rate with no mortality and only 4.8% of major complications in patient treated with slow (>6 h) infusion of recombinant tissue plasminogen activator (rt-PA) for prosthetic valve obstruction (PVO). Additionally, although the American Heart Association (AHA) guidelines in 2014 (15,16) and their European Society of Cardiology (ESC) equivalent (17) in 2012 assign to thrombolysis a grade of recommendation IIB in very high risk patient or to those in I or II New York Heart Association (NYHA) class with a thrombus size <0.8 cm, there is a tendency to accept thrombolysis as the first therapeutic line in most cases. In the PROMETEE (18) trial using slow t-PA infusion, the overall success rate was 90% with a single death (0.8%) in a NYAH IV patient, the complication rate was 6.7% and major embolism or bleeding occurred in 1.7% of the patients. In this study, a thrombus size <1 cm2 favourably correlated with the success of thrombolysis. Interestingly the average thrombus size on the aortic valves was 0.7 cm2 indicating that most of the valves in this condition may be suitable for the treatment.
Comparison with transthoracic and transesophageal echocardiography
Classically, PVO has been diagnosed by echocardiography, cinecardiography or a combination of the two. However, echocardiography still remains the first line tool to screen patients with suspected PVO. When the stroke volume is normal, Doppler evaluation of peak and mean transvalvular gradients should be compared to those published by the current guidelines for the brand and size. Besides the classic criteria for severe aortic stenosis (19) (peak velocity >4 m/s, mean peak gradient >40 mmHg, EOA <0.6 cm2/m2, acceleration time >100 ms), an acceleration time to ejection time ratio ≥0.4 has proved to be a reliable mark of PVO (20). When the conditions for severe PVO are not met, alternate imaging modalities should be considered. Whereas echocardiography can adequately identify thrombi or vegetations and quantify the degree of obstruction by Doppler examination, it may fail to identify the presence of pannus growing on the ventricular surface of aortic prosthetic valves. At this level, pannus can be easily masked by the prosthetic sewing ring, in fact as many as 40% of patients with pannus may show a normal prosthetic motion (21). It is therefore of the greatest importance to differentiate pannus formation from patient-prosthesis mismatch.
As an example, none of the malfunctioning mechanical aortic valves reported by Teshima in 2014 (22) had pannus demonstrated at echocardiography, whereas it was clearly visible at CT scan with attenuation values ranging from 151 to 183 HU. CT scan proved to be valuable also in moderate PVO as the extension and severity of the pannus measured as GOA (23) and encroachment ratio (21) correlate well with the degree of the prosthetic obstruction assessed by echocardiography.
Dealing with pannus
Whether pannus formation can be considered an exaggerated response to a foreign body (the prosthesis) remains unclear. Han in group of patients who had been submitted to AVR in the past and underwent cardiac CT, could detect pannus formation in 19%. Similarly, Suh (24) examined 92 patients with a mechanical aortic prosthesis and could find various degrees of pannus formation in 71 (77%); about half (32 pts, 45%) had at least mild extension, however only 12 pts required surgery because of valve obstruction. These data confirm that pannus is a common finding which in a limited number of cases, can impair the valve function.
Clinically, the accretion of pannus can go unnoticed until it affects the prosthetic function or causes significant obstruction (Figure 3) as demonstrated by Aoyagi (25) who examined 54 clinically asymptomatic patients later after AVR with the mechanical St Jude Medical prosthesis. Surprisingly at cineangiography, although all the valves showed a normal closure, 16 (29%) of them had an abnormal opening angle of 20° or more, 15 (27%) had intermediate values between 15° and 19° and only 23 (42%) had normal opening angles of 14° or less. As expected, the Doppler examination showed variable degrees of obstruction in the first group but gradients and flow velocities were normal in the remaining patients demonstrating that flow obstruction occurs only at an advanced stage. Eventually only five patients of the first group required valve replacement.
These observations pose a series of problems: how can we identify the patients at risk of pannus and whether the pannus formation is preventable or controllable.
The formation of pannus is a slow process; it is consistently reported that the PVO caused by pannus is evident only after several months after valve implantation. The average interval between the initial surgery and the detection of the pannus leading to reoperation was 10.5±5 years in the series reported by Ueda (9), 5.5±5.5 years in the report from Han (21) and 11.6±7 years in the series of Suh (24). No correlation has been found with the surgical technique or with inappropriate anticoagulation therapy. Conversely, patients with small body surface area (BSA) and small aortic annulus have shown a higher risk of severe obstruction, probably because even limited extension of pannus can cause a sizable reduction of GOA (12,20,23,24).
The spindle cells in the external medial layer (8) of the pannus express the Transforming Growth Factor-β-Receptor 1 (TGF-β-R1), whether this can be targeted by monoclonal antibodies to limit the production of collagen is debatable as is the use of steroids or nonsteroidal anti-inflammatory drug to limit the foreign body reaction that seems to promote the pannus formation.
Conclusions
Pannus is an uncommon but major late complication of valve replacement. In the aortic position, it should be suspected whenever a high gradient is seen in otherwise normal functioning prosthesis. When the transthoracic echocardiography can demonstrate a mass, some morphological, clinical features and CT scan are helpful in differentiating pannus from thrombus (Table 1). In fact, small thrombi with <90 HU are suitable for thrombolysis, especially in high risk patients. Currently, the cut-off attenuation for pannus is set at 145 HU on ECG-gated CT scans. Pannus is a slow growing tissue which in selected patients without severe PVO requires an individualized decision.

Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jacobs JP, Shahian DM, Prager RL, et al. The Society of Thoracic Surgeons National Database 2016 Annual Report. Ann Thorac Surg 2016;102:1790-7. [Crossref] [PubMed]
- Beckmann A, Funkat AK, Lewandowski J, et al. German Heart Surgery Report 2015: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2016;64:462-74. [Crossref] [PubMed]
- Grant SW, Hickey GL, Ludman P, et al. Activity and outcomes for aortic valve implantations performed in England and Wales since the introduction of transcatheter aortic valve implantation. Eur J Cardiothorac Surg 2016;49:1164-73. [Crossref] [PubMed]
- Kumar RK, Tandon R. Rheumatic fever & rheumatic heart disease: the last 50 years. Indian J Med Res 2013;137:643-58. [PubMed]
- Konen E, Goitein O, Feinberg MS, et al. The role of ECG-gated MDCT in the evaluation of aortic and mitral mechanical valves: initial experience. AJR Am J Roentgenol 2008;191:26-31. [Crossref] [PubMed]
- Gündüz S, Özkan M, Kalçik M, et al. Sixty-Four-Section Cardiac Computed Tomography in Mechanical Prosthetic Heart Valve Dysfunction: Thrombus or Pannus. Circ Cardiovasc Imaging 2015;8:e00324. [Crossref] [PubMed]
- Suchá D, Symersky P, Tanis W, et al. Multimodality Imaging Assessment of Prosthetic Heart Valves. Circ Cardiovasc Imaging 2015;8:e003703. [Crossref] [PubMed]
- Teshima H, Hayashida N, Yano H, et al. Obstruction of St Jude Medical valves in the aortic position: histology and immunohistochemistry of pannus. J Thorac Cardiovasc Surg 2003;126:401-7. [Crossref] [PubMed]
- Ueda T, Teshima H, Fukunaga S, et al. Evaluation of prosthetic valve obstruction on electrocardiographically gated multidetector-row computed tomography--identification of subprosthetic pannus in the aortic position. Circ J 2013;77:418-23. [Crossref] [PubMed]
- Hirsh J, Buchanan MR, Ofosu FA, et al. Evolution of thrombosis. Ann N Y Acad Sci 1987;516:586-604. [Crossref] [PubMed]
- Gündüz S, Karakoyun S, Kalçık M, et al. Multimodality imaging for solution of the old dilemma: Pannus or thrombus. J Cardiovasc Comput Tomogr 2016;10:e19-20. [Crossref] [PubMed]
- Moss AJ, Dweck MR, Dreisbach JG, et al. Complementary role of cardiac CT in the assessment of aortic valve replacement dysfunction. Open Heart 2016;3:e000494. [Crossref] [PubMed]
- Vitale N, Renzulli A, Agozzino L, et al. Obstruction of mechanical mitral prostheses: analysis of pathologic findings. Ann Thorac Surg 1997;63:1101-6. [Crossref] [PubMed]
- Özkan M, Gündüz S, Biteker M, et al. Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: the TROIA trial. JACC Cardiovasc Imaging 2013;6:206-16. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-e132. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [Crossref] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Özkan M, Gündüz S, Gürsoy OM, et al. Ultraslow thrombolytic therapy: A novel strategy in the management of PROsthetic MEchanical valve Thrombosis and the prEdictors of outcomE: The Ultra-slow PROMETEE trial. Am Heart J 2015;170:409-18. [Crossref] [PubMed]
- Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:589-90. [Crossref] [PubMed]
- Ben Zekry S, Saad RM, Ozkan M, et al. Flow acceleration time and ratio of acceleration time to ejection time for prosthetic aortic valve function. JACC Cardiovasc Imaging 2011;4:1161-70. [Crossref] [PubMed]
- Han K, Yang DH, Shin SY, et al. Subprosthetic Pannus after Aortic Valve Replacement Surgery: Cardiac CT Findings and Clinical Features. Radiology 2015;276:724-31. [Crossref] [PubMed]
- Teshima H, Aoyagi S, Ueda T, et al. Evaluation of advancing the standard valve dysfunction by multidetector-row CT. J Artif Organs 2014;17:162-8. [Crossref] [PubMed]
- Suh YJ, Kim YJ, Lee S, et al. Utility of cardiac computed tomography for evaluation of pannus in mechanical aortic valve. Int J Cardiovasc Imaging 2015;31:1271-80. [Crossref] [PubMed]
- Suh YJ, Lee S, Im DJ, et al. Added value of cardiac computed tomography for evaluation of mechanical aortic valve: Emphasis on evaluation of pannus with surgical findings as standard reference. Int J Cardiol 2016;214:454-60. [Crossref] [PubMed]
- Aoyagi S, Nishimi M, Kawano H, et al. Obstruction of St Jude Medical valves in the aortic position: significance of a combination of cineradiography and echocardiography. J Thorac Cardiovasc Surg 2000;120:142-7. [Crossref] [PubMed]


