What is the optimal first-line treatment for advanced anaplastic lymphoma kinase-rearranged non-small cell lung cancer?
Anaplastic lymphoma kinase (ALK)-gene rearrangements work as an oncogenic driver in 3–8% of patients with non-small cell lung cancer (NSCLC) (1,2). These patients tend to be younger than those without driver mutations, and have no or little smoking history. ALK-rearranged tumors are usually adenocarcinomas, frequently with an acinar-predominant structure (3). In general, ALK rearrangements are mutually exclusive of other activating mutations such as epidermal growth factor receptor (EGFR) and KRAS mutations (2).
Crizotinib, the first ALK-tyrosine kinase inhibitor introduced clinically, showed a dramatic tumor response in 61% of patients and a 1-year survival rate of 75% in heavily treated patients with ALK-rearranged NSCLC (4,5). Furthermore, a phase III trial in the first-line setting (PROFILE1014) demonstrated a clinically and statistically significant improvement in the median progression-free survival (PFS) [95% confidence interval (CI)] while using crizotinib when compared with conventional platinum and pemetrexed chemotherapy [10.9 months (8.3–13.9 months) versus 7.0 months (6.8–8.2 months) at a hazard ratio (HR) of 0.45 (95% CI, 0.35–0.60)] (6). In most patients, however, the tumor ultimately progresses with enlargement in the primary site and development of metastases, especially to the brain, which is the most common site of progression. The main mechanisms of this acquired resistance during crizotinib treatment are target alteration (mutations in the ALK kinase domain and amplification of the ALK fusion gene) in 30–50% of tumors and alterative pathway activation via genes, including EGFR, KRAS, KIT, and insulin-like growth factor 1 receptor (IGF1R), in approximately 30–40% of tumors (2,7-10).
Because as many as 50% of crizotinib-resistant tumors are considered ALK-pathway dependent, second-generation ALK-tyrosine kinase inhibitors have been developed to enhance anti-ALK activity. These agents effectively inhibit the growth of tumor cells with crizotinib-resistance mutations in vitro (Table 1) (11-14). Ceritinib is a potent ALK-inhibitor that has inhibitory effects on both IGF1R and insulin receptor 1 (15). A phase I study of ceritinib in an expansion cohort of patients with ALK-rearranged NSCLC (ASCEND-1) showed that objective responses were noted in 72% of patients untreated with any ALK inhibitor and in 56% of patients pretreated with an ALK inhibitor (16). A phase II study of patients who had received cytotoxic chemotherapy and had shown disease progression during crizotinib treatment (ASCEND-2) revealed a response rate of 38.6% (17). Alectinib is another ALK-inhibitor that is highly promising against crizotinib-resistant NSCLC. Two phase II trials for crizotinib-refractory ALK-rearranged NSCLC showed an objective response rate of 48–50% after treatment with alectinib (18,19). Brigatinib (AP26113) is effective against a broad range of ALK genes with second mutations including G1202R that confer resistance against crizotinib, ceritinib, and alectinib (Table 1). Brigatinib also inhibits mutant EGFR, including L858R and L858R/T790M (14). Brigatinib was associated with the highest response rate of 62% among patients with crizotinib-resistant NSCLC (20).
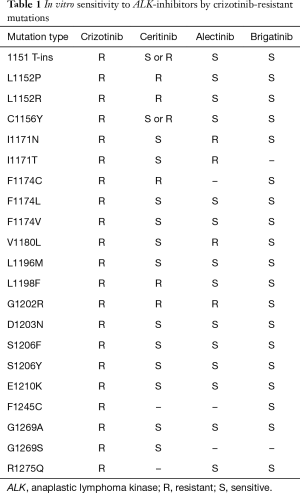
Full table
Interestingly, ALK-resistant mutations were present in 56% of patients who received a second-generation ALK-inhibitor, whereas such mutations were found in only 20% of patients who received crizotinib (P=0.0002). This indicates that inadequate suppression of ALK may paradoxically induce the alterative pathway activation, resulting in tumors that are currently difficult to treat (9). Thus, a more complete suppression of ALK with a second-generation ALK-inhibitor from the start of treatment may be important to improve patient survival.
Soria et al. reported the results of a phase III trial of first-line ceritinib versus platinum and pemetrexed chemotherapy for patients with advanced non-squamous ALK-rearranged NSCLC (ASCEND-4) (21). The primary endpoint was PFS assessed by a blinded independent review committee. Under the assumption that the median PFS was 8 and 13 months in the chemotherapy and ceritinib arms, respectively, 205 PFS events were required to have 90% power at a one-sided 2.5% level of significance, in order to reject the null hypothesis. The sample size was finally determined to be 348 patients, estimating a recruitment period of 32 months and a dropout rate of 15%. This study actually included 376 patients with ALK-rearranged NSCLC who received no systemic anticancer therapy. They were randomized to receive either 750 mg/day ceritinib daily (n=189) or intravenous chemotherapy [75 mg/m2 cisplatin or carboplatin (target area under the curve of 5–6) plus 500 mg/m2 pemetrexed] repeated every 3 weeks (n=186). Both treatments were well tolerated; most toxicities were grade 1–2 in severity. Grade 3–4 toxicities were observed in less than 10% of patients, except for liver dysfunction in 30% of patients in the ceritinib arm and neutropenia in 11% of patients in the chemotherapy arm. The objective response rate (95% CI) assessed by the independent review committee was 72.5% (65.5–78.7%) in the ceritinib arm and 26.7% (20.5–33.7%) in the chemotherapy arm. The median (95% CI) duration of response was 23.9 months (16.6 months to not estimable) in the ceritinib arm and 11.1 months (7.8–16.4 months) in the chemotherapy arm. The median (95% CI) PFS was 16.6 months (12.6–27.2 months) and 8.1 months (5.8–11.1 months) in the ceritinib and chemotherapy arms, respectively, with a HR of 0.55 (95% CI, 0.42–0.73). This benefit in the ceritinib arm was obtained across most subgroups with different patient characteristics. In patients with brain metastasis (n=121), the median (95% CI) PFS in the ceritinib and chemotherapy arms were 10.7 months (8.1–16.4 months) and 6.7 months (4.1–10.6 months), respectively, with a HR of 0.70 (95% CI, 0.44–1.12). Among the patients who discontinued chemotherapy (n=145), 105 (72%) received an ALK inhibitor. Of these, 80 (55%) patients received ceritinib. The overall survival data were immature; the median overall survival was not reached in the ceritinib arm and was 26.2 months in the chemotherapy arm, with a HR of 0.73 (95% CI, 0.50–1.08, P=0.056). The estimated overall survival rates (95% CI) at 24 months were 70.6% (62.2–77.5%) in the ceritinib arm and 58.2% (47.6–67.5%) in the chemotherapy arm. These results clearly showed that this study met the primary objective.
Alectinib as a first-line treatment was also evaluated in a phase III trial in comparison to crizotinib (J-ALEX) (22). A total of 207 patients with advanced ALK-rearranged NSCLC were randomized and treated with either 300 mg alectinib twice daily or 250 mg crizotinib twice daily until disease progression or unacceptable toxicity. The objective response rate (95% CI) was 91.6% (85.6–97.5%) and 78.9% (70.5–87.3%), respectively, in the alectinib and crizotinib arms. The median (95% CI) PFS, the primary endpoint of this study, was not reached yet (20.3 months to not estimable) in the alectinib arm versus 10.2 months (8.2–12.0 months) in the crizotinib arm, with a HR of 0.34 (99% CI, 0.17–0.71). Two phase III trials with the same design are in progress in the world other than Asia (ClinicalTrials.gov Identifier, NCT02075840) and in China, Korea, and Thailand (ClinicalTrials.gov Identifier, NCT02838420).
These phase III trials evaluating crizotinib, ceritinib, and alectinib revealed that the median PFS after treatment with chemotherapy or crizotinib was stable, approximately 7–8 and 10–11 months, respectively. When compared with these efficacy data, the results with second-generation ALK-inhibitors were promising; the median PFS for these agents was more than 16 months (Table 2) (6,21,22). In addition, a phase III trial of brigatinib versus crizotinib (ALTA-1L) is under way in patients with ALK-rearranged advanced NSCLC who had never received any ALK-inhibitors (ClinicalTrials.gov Identifier, NCT02737501). Thus, comparative trials may be necessary among second-generation ALK-inhibitors.

Full table
Although overall survival is quite an important indicator of anti-cancer therapy with a new agent, it is very difficult to evaluate the association between the first-line agent and overall survival in this setting. The post-progression survival can be more closely correlated to overall survival compared with PFS after first-line therapy (23). However, because it is influenced by multiple agents used in the second- or later-lines of therapy as well as supportive care in clinical practice, post-progression treatment generally cannot be controlled so as to be kept comparable between the treatment arms. There is accumulating evidence that the choice of anticancer agents during the post-progression period should be based on the results of re-biopsy of the tumors (24). The development of liquid biopsy will facilitate this strategy in clinical practice.
Acknowledgements
The author would like to thank Editage (www.editage.jp) for English language editing.
Footnote
Conflicts of Interest: Honoraria—Chugai Pharmaceutical Co., Novartis Pharma, and Pfizer.
References
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013;19:4273-81. [Crossref] [PubMed]
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009;22:508-15. [Crossref] [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med 2012;4:120ra17. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res 2015;21:2227-35. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011;19:679-90. [Crossref] [PubMed]
- Kodama T, Tsukaguchi T, Yoshida M, et al. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett 2014;351:215-21. [Crossref] [PubMed]
- Zhang S, Anjum R, Squillace R, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res 2016;22:5527-38. [Crossref] [PubMed]
- Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl) phenyl) -N4-(2-(isopropylsulfonyl) phenyl) pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem 2013;56:5675-90. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Crinò L, Ahn MJ, De Marinis F, et al. Multicenter phase II study of whole-body and Intracranial Activity With Ceritinib in Patients with ALK-rearranged non-small-cell lung cancer previously treated with Chemotherapy and Crizotinib: results from ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683-96. [Crossref] [PubMed]
- Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Nokihara H, Hida T, Kondo M, et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK + NSCLC): Primary results from the J-ALEX study. J Clin Oncol 2016;34:abstr 9008.
- Hotta K, Kiura K, Fujiwara Y, et al. Role of survival post-progression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: a systematic review. PLoS One 2011;6:e26646. [Crossref] [PubMed]
- Ou SH, Milliken JC, Azada MC, et al. ALK F1174V mutation confers sensitivity while ALK I1171 mutation confers resistance to alectinib. The importance of serial biopsy post progression. Lung Cancer 2016;91:70-2. [Crossref] [PubMed]


