The emerging role of YAP/TAZ in mechanotransduction
Cells sense their physical surroundings through mechanotransduction—that is by translating mechanical forces and deformations into biochemical signals that can ultimately influence gene expression, cell shape and cell fate. Recently, YAP/TAZ—the main transcriptional effectors of the Hippo signalling pathway, a major growth regulatory pathway within metazoa—have been identified as key mechanotransducers acting by nuclear relays of mechanical stimuli (1).
YAP/TAZ nuclear accumulation and activation has been associated with organ growth, increase in cell size, loss of contact inhibition and tumour growth, cellular proliferation and inhibition of apoptotic signals (1,2). The emergence of these functions have been realized through binding to TEAD, SMAD1/2/3, PPARγ, TBX5 and TTF1 where YAP/TAZ acts as a transcriptional co-factor, thereby regulating >400 genes (1,2).
In mammals, the Hippo pathway consists of a cascade of interacting kinases (MST 1/2, LATS 1/2), which eventually phosphorylate YAP/TAZ at the serine-127 (S127) residue. Phosphorylation of S127 of YAP/TAZ is considered an exclusion signal for nuclear accumulation, leading to ubiquitination (1,3,4). Hence, activation of the Hippo pathway leads to inhibition of YAP/TAZ proteins. Upstream regulators of the Hippo pathway are Merlin, which is encoded by NF2. In confluent cells, Merlin/NF2 are preferentially localised in close proximity to adherens and tight junctions, and this location has been associated with the known activation of the Hippo pathway and inhibition of YAP/TAZ in cell-cell contact inhibition (1,3,4).
YAP/TAZ is a known sensor for mechanical stimuli including matrix stiffness, stretch and cell density. Sophisticated experiments from the Piccolo’s lab as well as others have examined YAP/TAZ activation after altering cellular or extracellular mechanics including altering F-actin accumulation, plating cells on stiff versus soft substrates, exposing cells to cyclic stretching, modulating the extent of cellular attachment to the extracellular matrix (ECM) and altering cell shape through varied ECM geometries. Furthermore, YAP/TAZ nuclear accumulation and activation has been associated with organ growth, increase in cell size, loss of contact inhibition and tumour growth, cellular proliferation and inhibition of apoptotic signals. However, the influence of other mechanical factors such as shear forces and haemodynamic stimuli on YAP/TAZ activity requires further investigation (5-8).
Following from the above experiments and in order to answer the question as to whether YAP/TAZ activation by mechanical forces is regulated by the canonical Hippo pathway, further investigations were carried out. LATS 1/2 dependent phosphorylation of S127 was evaluated and not upregulated by mechanical cues. Additionally, inhibition of LAST 1/2 had only a marginal effect on mechanical regulation of YAP/TAZ (5). Finally, in confluent cells where the Hippo pathway inhibits YAP/TAZ through contact inhibition, re-activation of YAP/TAZ was possible by stretching (8). These data suggest that YAP/TAZ induction by mechanical cues is independent of and acting in parallel to the Hippo pathway.
One wonders if there is a parallel path for each mechanical cue or just one common denominator. Clarification of this statement coincides with a long-lasting discussion in the field of mechanobiology as to whether mechanical signals are perceived by the cell through the cytoskeleton (“Central Mechanism”), via a combination of mechanosensors (“Peripheral Mechanism”) or both (9). Experimental evidence has been generated supporting all theories, whereby the application of external forces (“shear stress” and “stretch”) often are interpreted in the context of the activation of mechano-sensors while cell spreading/cell-matrix interactions often are explained by internal stress on the cytoskeleton. In reality, both systems are intertwined and influence each other.
The cell matrix/substrate experiments favour a role for RhoA and fibre tension in YAP/TAZ control. In addition, YAP/TAZ binds to E-cadherin and JAMS that are believed to be involved in mechanosensing (2,5,6,10,11). Their binding to the cytoskeleton is essential for the unfolding of the proteins after the stretch of the cytoskeleton or the stretch of lipid bilayer by external forces.
Atherosclerosis can be viewed as a disease of altered mechanotransduction. Atherosclerotic lesions are often found at specific sites of disturbed flow patterns, characterised by low and oscillatory shear stress at the endothelium, while unidirectional laminar shear stress is regarded as having an atheroprotective effect. Upregulation of some YAP/TAZ target genes in atherosclerotic plaques and the recent discovery that YAP/TAZ is under the metabolic control of the mevalonate pathway with statins (anti-atherosclerotic drugs) that are strong YAP inhibitors are indicators of the involvement of YAP/TAZ in atherogenesis. However, direct links between altered haemodynamics, YAP/TAZ activity and atherosclerosis are yet to be investigated.
Through a series of compelling in vitro and in vivo experiments, Wang et al. examined the roles of YAP/TAZ haemodynamics-induced mechanotransduction and pathogenesis of atherosclerosis, and found that endothelial YAP/TAZ activation is induced by disturbed flow (commonly found in atheroprone regions), which in turn promotes atherogenesis via enhancing JNK-mediated upregulation of pro-inflammatory gene expression (1,11). In stark contrast, unidirectional shear stress (USS) was associated with an atheroprotective effect and was found to inhibit YAP/TAZ activity by modulating the β3 integrin-Gα13-RhoA pathway. In vivo endothelial-specific YAP/TAZ knockdown (via CRISPR/Cas-9) or MnCl2 treatment (strong activator of β3 integrins) in apolipoprotein E-deficient (ApoE-/-) mice (a common model of atherogenesis) delayed plaque formation and atherogenesis, and conversely constitutive in vivo activation of endothelial YAP/TAZ accelerated atherogenesis (Figure 1). Seeking whether existing anti-atherosclerotic drugs inhibit YAP/TAZ activity, the group tested a panel of several agents and found that in addition to statins, apelin, ApoA1 and niacin also suppressed YAP/TAZ activity. Furthermore, the anti-inflammatory effect of statins was confirmed to be secondary to YAP/TAZ inhibition, as constitutive activation of YAP/TAZ in HUVECs failed to suppress expression of pro-inflammatory genes under statin treatment. Thus, the mechanoresponsive integrin-Gα13-RhoA-YAP pathway holds promise as a novel drug target against atherosclerosis (Figure 1).
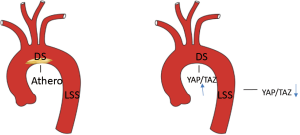
In conclusion, YAP/TAZ is possibly a central regulator for flow-induced atherosclerosis with potential therapeutic effects. There remain some unanswered questions that require further investigation: what are the characteristics of the substrate under endothelial cells that are exposed to disturbed flow? Is activation of YAP/TAZ associated with endothelial apoptosis and enhanced lipid uptake in the vessel wall? What is the role of other known signalling pathways interacting with YAP/TAZ such as the Wnt pathway that was recently shown to be regulated by blood flow (12-14)?
Acknowledgements
Funding: The financial support of the British Heart Foundation (RG/11/13/29055, PG/15/49/31595 and SP/17/1/32702) is greatly acknowledged.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014;94:1287-312. [Crossref] [PubMed]
- Janmey PA, Wells RG, Assoian RK, et al. From tissue mechanics to transcription factors. Differentiation 2013;86:112-20. [Crossref] [PubMed]
- Santucci M, Vignudelli T, Ferrari S, et al. The Hippo Pathway and YAP/TAZ-TEAD Protein-Protein Interaction as Targets for Regenerative Medicine and Cancer Treatment. J Med Chem 2015;58:4857-73. [Crossref] [PubMed]
- Guo L, Teng L. YAP/TAZ for cancer therapy: opportunities and challenges Int J Oncol 2015;46:1444-52. (review). [PubMed]
- Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature 2011;474:179-83. [Crossref] [PubMed]
- Wang KC, Yeh YT, Nguyen P, et al. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A 2016;113:11525-30. [Crossref] [PubMed]
- Bertero T, Oldham WM, Cottrill KA, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 2016;126:3313-35. [Crossref] [PubMed]
- Pathak MM, Nourse JL, Tran T, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A 2014;111:16148-53. [Crossref] [PubMed]
- Davies PF, Dewey CF Jr, Bussolari SR, et al. Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest 1984;73:1121-9. [Crossref] [PubMed]
- Chanet S, Martin AC. Mechanical force sensing in tissues. Prog Mol Biol Transl Sci 2014;126:317-52. [Crossref] [PubMed]
- Wang L, Luo JY, Li B, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Zhang K, Qi HX, Hu ZM, et al. YAP and TAZ Take Center Stage in Cancer. Biochemistry 2015;54:6555-66. [Crossref] [PubMed]
- Piersma B, Bank RA, Boersema M. Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front Med (Lausanne) 2015;2:59. [Crossref] [PubMed]
- Xu F, Zhang J, Ma D. Crosstalk of Hippo/YAP and Wnt/β-catenin pathways. Yi Chuan 2014;36:95-102. [Crossref] [PubMed]


