Wrapping of the ascending aorta revisited—is there any role left for conservative treatment of ascending aortic aneurysm?
Introduction
The conservative treatment of aortic aneurysms precedes the surgical removal of the aorta and its replacement with homologous or synthetic grafts. Reverse remodeling of the affected aorta—aneurysmorrhaphy—with external suturing or wrapping with different synthetic materials (cellophane, polyethylene, etc.) was first carried out by Matas on the abdominal aorta and later by Tuffier on the thoracic aorta (1).
Advances in surgical techniques and the development of new materials made it possible to completely remove the affected aorta and replace it with biological or synthetic conduits. Over time, the surgical risk associated to replacement of the ascending aorta (RAA) substantially decreased, and the long-term evolution revealed long survival with a low incidence of related complications. According to the results from a recent review carried out in the United States, early mortality for elective replacement of the ascending aorta (AA) is 3.4% (2). However, this figure increase significantly when the procedure is performed in elderly patients with multiple comorbidities, ranging from 4% to 20% (3). In these cases, or when the correction of the aortic pathology is associated with other procedures, a conservative surgical approach for the AA aneurysm may be beneficial, because it is simpler and involves a lower risk.
In 5–15% of the patients requiring surgery of the aortic valve exists a moderate dilatation—40 to 50 mm—of the AA (4). The approach in these cases is controversial. Some observational studies have warned about the risk of acute aortic complications in these patients (5-7). According to them, rupture and/or dissection of the AA are frequent after isolated valve surgery, and they often appear in patients with an AA size lower than indicated for replacement. Conversely, other more recent studies have not confirmed these fears and conclude that the risk of such complications is low (8). Lee et al. have documented an incidence of adverse aortic events—rapid growth or dissection—of 0.5% per patient-year (9). Under these circumstances, some surgeons merely treat the valve pathology on the assumption that correcting this pathology will stop the progressive dilatation of the AA. On the contrary, other surgeons adopt some strategies to prevent dilatation, particularly in patients with a bicuspid aortic valve.
Conservative surgical options
The two surgical options, which make possible to correct aneurysms of the AA without having to replace it, are reduction aortoplasty and external remodeling with an prosthesis or wrapping.
After some sporadic and little successful attempts carried out in the 50s and 60s, Francis Robicsek refined and reintroduced reduction aortoplasty at the end of the 70s as a less invasive alternative to RAA (10). Originally, this technique involved the resection of an oval section of the anterior aortic wall with direct suture of the wall defect in an attempt to reestablish an aortic diameter of less than 35 mm. Afterwards, several modifications have been described regarding the methods of aortic incision, although their potential benefits have not been proven. Alternatively, the same effect may be achieved through the plication of a segment of the anterior aortic wall, equivalent to the one that should have been resected, along Teflon strips. Although this technique is simple and may be performed with a low surgical risk, the fact that the patient is left with a diseased aorta involves a risk of re-dilatation or other acute aortic complications. For this reason, unsupported aortoplasty has been nearly abandoned and it is recommended to complement it with wrapping of the AA with some type of material (11). In these circumstances, wrapping does not only aim to reduce the diameter of the aorta, but also to reinforce the wall and prevent subsequent dilatation.
Reduction aortoplasty has been used, normally associated with other procedures, in small series, generally with mildly dilated aortas and in patients without connective tissue diseases. It is a less aggressive technique than RAA, it requires less ischemia time and it has lower morbidity and mortality rates. The long-term results are generally acceptable, with a low incidence of aortic re-dilatation (11-13). However, no prospective studies have been carried out to compare its results with those of other techniques. On the other hand, some cases of rupture of the remaining aortic wall have been reported, in spite of the external synthetic reinforcement (14). The incidence of this complication was 1.1% in a survey carried out by Robicsek in 2004 (15).
The other conservative procedure is wrapping to reduce the AA, which is even older. Ake Senning introduced this technique in the 60s, using a polypropylene mesh, although the results were not published until 1982 (16). The technique involves the use of a vascular prosthesis with a preselected diameter, shorter than that of the AA, in order to reduce the AA diameter and reinforce the aortic wall, thus preventing its dilatation and the need of additional surgical procedures on the aortic wall. Therefore, the surgical technique is simplified, the myocardial ischemia time is reduced and the potential complications associated with aortotomy are prevented. In spite of its apparent technical simplicity, this procedure has been brought into question, mainly due to the possible negative consequences it may have on the aortic wall.
Wrapping technique
Access to the heart and the aorta may be achieved through conventional or reduced sternotomy. In any case, it is necessary to completely dissect the AA, from the sinotubular junction (STJ) to the origin of the brachiocephalic artery (BCA), separating it from the pulmonary artery and releasing the posterior pericardial reflection. This dissection is more easily performed after starting cardiopulmonary bypass (CPB), once the pulmonary artery is decompressed.
The objective of wrapping is to reduce the diameter of the aorta to normal dimensions for the size of the patient, and in any case to less than 35 mm in diameter. This target may be achieved with different biological or synthetic materials. Generally, a straight polyester vascular graft of 32 or 34 mm is used, and a low-porosity prosthesis is not necessary, since it will not be in contact with the bloodstream. In fact, the low porosity of conventional vascular prostheses may theoretically be an inconvenient, because it promotes the accumulation of fluids between the prosthesis and the aortic wall, which would make it difficult to achieve good adherence between both structures (17). On the contrary, it is important to use an elastic graft that may mimic the normal compliance of the aorta. Additionally, meshes made up of different synthetic materials can be used, including polyester, polypropylene or polytetrafluorethylene. In this case, it is necessary to size them beforehand to achieve an adequate aortic diameter (18-20). Unlike vascular prostheses, whose maximum diameter is 34 or 36 mm, depending on the manufacturer, meshes may be manipulated to achieve a larger final aortic diameter, which makes it possible to use them on aortas up to 70 mm in diameter (19). Also, thanks to their elasticity, they adapt better to the aortic contour and alow some systolic expansion.
Afterwards, the necessary cannulations are performed to establish the CPB. In order to be able to place the wrapping during the final stages of the CPB without the aortic cannula as a determining factor, it is advisable to place the aortic cannula in the initial segment of the aortic arch. Alternatively, some authors prefer to use peripheral arterial cannulation, either in the femoral artery or in the axillary artery (20).
Once the AA has been cross-clamped, the aortic valve procedure is performed, accessing the valve through a transverse aortotomy performed 1 cm above the STJ. The inferior lip of the aortotomy is retracted inferiorly with two 4-0 polypropylene sutures placed at the two commissures of the right coronary cusp, and buttressed with Teflon pledgets on the inner side. These sutures will be later used in the proximal fixation of the vascular graft.
Wrapping is performed after aortic declamping, during the rewarming time. This makes it easier to control arterial pressure and to place the external prosthesis. The preselected vascular graft is trimmed to the desired length and with beveled ends so that the graft can adapt to the shorter length of the lesser aortic curvature. The length of the graft used must be equal to the distance from the STJ to the origin of the BCA. This measurement must be carried out with a slightly stretched graft in order to reduce the marks left by the corrugated material, as in the case of RAA. It is recommended that the final graft length should be slightly larger than the aortic segment that is going to be reinforced.
Afterwards, the prosthesis is opened longitudinally along some of the reference marks, and it is placed around the AA. Next, approximating the sectioned edges reduces the diameter of the aorta. This maneuver can be facilitated by approximating the four corners of the open prosthesis with Crile forceps while 2-0 TiCron sutures are applied in both ends, in the middle point and in the two intermediate points. It is important to cover the entire AA and to let the graft settle regularly—like a glove—so that it is not excessively stretched and there is no redundant material. In the first case, transmural fixation sutures may be subject to excessive tension, with the subsequent risk of rupture of the aortic wall. In the second case, the graft may form folds that compress the aorta, which involves a higher risk of aortic wall erosion. The formation of folds may be enhanced by a marked curvature of the aorta and by the need of a greater reduction of the aortic diameter, as in the cases in which it is over 50 mm. Vascular grafts are straight and have a maximum diameter of 36 mm, although their corrugated design allow them some curvature without creating folds. In order to prevent the formation of folds it is essential to adequately measure the necessary length of the graft and to apply the correct longitudinal tension. As an alternative to a straight graft, Tappainer et al. proposed the creation of a slightly curved tubular support made up of two segments of a straight vascular prosthesis (21).
Next, the approximation of the graft is secured with a double 4-0 polypropylene suture that begins at the distal portion, where it can be supported by the aortic adventitia, and it is reinforced by a Teflon pledget in order to prevent any potential displacement. While this double suture is being placed, the aortic wall must not be perforated, and it is important to keep the vascular graft stretched in order to ensure an adequate placement. To do so, it is advisable to pull both ends of the TiCron approximating sutures placed on the upper and lower edges in opposite directions, and to refrain from cutting those ends until approximation of the prosthesis has been completed. Initial operative appearance, intermediate step of the procedure and final result are shown in Figure 1.
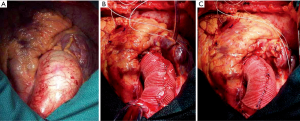
Another crucial aspect is the proper fixation of the prosthesis in both the proximal and distal ends. In order to ensure an adequate coverage of the entire AA, the prosthesis must be fixed on the STJ and it must cover the aortotomy. As Carrel et al. (4) suggest it is advisable to fix the prosthesis at the level of the three commissures and at the middle of the non-coronary sinus, where it tends to retract more. The safest way to do it is with double 4-0 polypropylene sutures buttressed with small Teflon pledgets inside the aorta and on the vascular prosthesis outside.
Whenever it is necessary to perform an associated myocardial revascularization, it is advisable to use arterial grafts that are not connected to the AA. If free grafts are used, such as the radial artery or the saphenous vein, the proximal anastomosis can be carried out on unreinforced aortic segments, such as the root—undesirable—the BCA or the aortic arch. Anastomosis can also be performed on the reinforced segment of the AA after a fenestration of the prosthesis, as we have done in our group occasionally. However, this option is not advisable due to the risk of leaving an unprotected aortic area, which may further dilate or compress the aorto-coronary anastomosis if the vascular prosthesis is displaced.
Although in most cases wrapping of the AA is associated with the correction of other cardiac pathologies, it has also been performed as an isolated procedure, in which case it can be carried out without the need of a CPB (19).
Biomechanical effects
The biomechanical objective of wrapping is the reduction of the AA diameter. According to Laplace’s law, this effect reduces the stress of the aortic wall. Also, the external reinforcement increases the thickness of the aortic wall. Both measures contribute to prevent dilatation and the appearance of a dissection or rupture of the AA (22,23). When the wrapping is performed correctly, it reduces the AA diameter to the preselected size. Also, wrapping reduces the size of the STJ and the sinuses of Valsalva and, to a lesser extent, the diameter of the proximal section of the aortic arch. This reverse remodeling of the aorta becomes evident immediately after the operation, and it persists over time. In a recent study by Plonek et al., the maximum diameter of the aorta decreased by 39%, from an average value of 50.5 mm down to 30.7 mm (24). The final diameter of the AA was, on average, 4.5 mm shorter than the diameter of the vascular prosthesis what had been used. When the reduction in the size of the AA is excessive, as in the case of aneurysms with a diameter over 55–60 mm, wrapping may lead to aortic wall deformation by creating longitudinal folds. This decrease leaves a free space, which is occupied by thrombi and pericardial fluid, and it is shown as a covering layer of variable thickness that may be mistaken for an intramural hematoma in postoperative images.
However, as in the case of RAA, wrapping of the AA also modifies the biomechanical characteristics of the blood flow in the entire arterial system (25). The synthetic materials used to externally reinforce the aorta are much less elastic than a healthy or diseased native aorta, which alters the pressure of the entire arterial system. This fact has been associated with a higher risk of cardiovascular events in hypertensive patients (26). On the other hand, the compliance mismatch between the native and the reinforced aorta leads to hemodynamic changes that promote intimal hyperplasia and increase wall stress in the aortic segments closest to the reinforced section (25).
The effects on the distribution of wall stress after wrapping have recently been studied by Plonek et al. through advanced computational analyses based on the finite elements method (27). Measurements were taken on a normal aorta (32 mm) and a moderately dilated one (45 mm) under physiological hemodynamic and mobility conditions. Wall stress magnitude and distribution in the wrapped aorta were only slightly higher than those of the normal aorta, and much lower than those of the moderately dilated aorta, both on the outer and the inner surface. The recorded stress on the inner surface of the aorta was even lower in the externally reinforced aorta than in the non-dilated one, which may imply a lower risk of its dissection. As in the case of the non-dilated aorta, the highest stress in the wrapping model was noticed on the distal part, at the junction between the AA and the aortic arch. Another mathematical model has shown additional advantages on the aorta and the ventricular afterload when the AA diameter is reduced with elastic material with high compliance (28).
Structural or histologic effects
One of the main criticisms of wrapping has been the possibility to induce a degeneration or atrophy of the aortic wall, which may lead to subsequent complications. This effect would be a consequence of the constant compression caused by the foreign material used to reduce its diameter, which also prevents normal expansion during systole. The abnormal macroscopic image of the reinforced aorta, which may even disappear in some specific areas, has been described during reoperation of patients who had undergone wrapping (29), and we can confirm it as well.
Neri et al. carried out an histologic examination of aortic tissue specimens retrieved from two patients during a reoperation performed years after they had undergone supported reduction aortoplasty, and compared them with the samples retrieved during the first operation (14). These authors described that the reinforced aorta was significantly thinner, with a sclerotic microstructure in which layers were no longer present. Also, the atrophied aortic wall showed cellular and neovascular infiltration and common in a foreign-body reaction. On the contrary, the samples retrieved from the non-reinforced aorta showed a basically normal histologic structure. Different mechanisms have been cited as being responsible for these changes in the microstructure of the aortic wall. They include the compromise of the vasa vasorum that nourish the middle layer of the aorta, chronic inflammatory response to the foreign material, and sustained compression of the aortic wall between the pressure of blood and of the external prosthesis. As a consequence of these changes, the aorta would become a passive conduit with biomechanical characteristics similar to those of a synthetic vascular prosthesis. These changes seem to be less patent when the AA is reinforced with a Dacron mesh instead of with a vascular conduit (18), probably due to its greater elasticity.
In any case, there is no evidence that the changes described on the structure of the reinforced aortic wall are associated with a higher risk of dissection or rupture.
Clinical results
The short- and long-term results of wrapping, associated or not with reduction aortoplasty, have been reported in several publications, generally based on a limited number of patients. In addition, there are no prospective studies that compare the results of wrapping with those of RAA. Also, we have only found a recent meta-analysis in which Plonek et al. analyze the results of wrapping, with and without reduction aortoplasty, in 722 patients (30). Only 4 out of the 17 studies included in this systematic review refer to patients—272— in which wrapping was performed alone, without an associated aortoplasty. On the other hand, in most patients (87%), wrapping was associated with an aortic valve operation.
Early results
The association of AA wrapping with aortic valve replacement does not increase early mortality or the incidence of major postoperative complications (9,22,23,31-33). Hospital mortality in the more than 700 patients included in the meta-analysis by Plonek was only 1.5%, and the deaths were unrelated to the surgical technique (30). No mortality was recorded on patients who underwent isolated wrapping or wrapping associated with AA plication, whereas it increased to 2% in patients who underwent concomitant aortoplasty with resection of the aortic wall. However, the study does not specify whether this may have been due to other factors apart from the surgical technique.
Compared with RAA, wrapping significantly reduces myocardial ischemia and CPB time, duration of the operation and need for a transfusion, which should involve a lower incidence of perioperative complications. Already in the 90s, Carrel et al. proved that the conservative treatment of AA aneurysms in patients with aortic valve disease led to lower mortality, incidence of perioperative myocardial infarction, neurological complications and reoperation for bleeding than RAA (4). In a more recent study, Zhang et al. found that the use of blood products and the duration of postoperative hospital stay were also lower in patients who underwent wrapping than in those who underwent AA replacement (33). These findings have been confirmed in a recent meta-analysis of five studies that compare the results of wrapping and RAA (30). On the contrary, Lee et al. did not find differences regarding mortality or the incidence of major postoperative complications between both techniques (9). Only the incidence of reoperation for bleeding was slightly higher in patients who underwent AA replacement (5.7%) than in those who underwent wrapping (1.5%).
These results are similar to those in our group, in which this technique has been used in 138 patients. In 84 of them, wrapping was concomitant with isolated aortic valve surgery. We compared the early results from this group with a similar one of 94 patients in which aortic valve surgery was combined with RAA. Wrapping made it possible to significantly reduce ischemia time (62 vs. 92 minutes), CPB time (88 vs. 122 minutes), the duration of the operation (185 vs. 230 minutes) and postoperative bleeding (580 vs. 850 mL). However, no significant differences were found regarding mechanical ventilation time (6 hours in both groups), the duration of stay in the Postoperative Care Unit (41 vs. 45 hours) or the time of postoperative hospital stay (8 days in both groups). No deaths were recorded in the wrapping group and two patients died in the RAA group (P=0.178).
Long-term results
There is no evidence to suggest that AA wrapping involves a higher risk of mortality or complications of the aorta in the long term. Lee et al. reported that the five-year survival rate in patients who underwent wrapping and aortic valve replacement (91.8%) was similar to that of those who only had an aortic valve replacement (90.1%) or to those with aortic valve and AA replacement (82.2%) (9). In this study, none of the patients receiving wrapping developed aortic aneurysm or an acute aortic complication, unlike 2 of the patients in which the aorta was not treated. Zhang et al. did also not find differences in the 4-year survival rate between patients who underwent wrapping (90.7%) and RAA (87.0%) (33). In another study with a longer follow-up period, Choi et al. found that the 10-year freedom from cardiac-related death was similar in patients with bicuspid aortic valve in which only the valvular disease was corrected (91.7%) and in those who also underwent AA wrapping (89.3%) (32). Only 2 out of the 722 (0.3%) patients included in the meta-analysis carried out by Plonek, in which the average follow-up time was 5 years, died due to complications related to the aorta, one due to an acute dissection and the other during the reoperation to treat the re-dilatation of the aorta (30). However, some studies describe cases of sudden death in patients in which no measures were taken on the AA, and this did not happen in patients who also underwent AA wrapping (9,32).
The decrease in the caliber of the AA achieved with wrapping remains stable in the long term (19,31,32). Over time, both the aortic arch and, particularly, the sinuses of Valsalva tend to become dilated, although not significantly, both after wrapping and after RAA. Zhang et al. revealed that the dilatation of the aortic root was slightly more marked after replacement than after wrapping (33). Consequently, the need to reoperate on patients who undergo AA wrapping for re-dilatation of the aorta is low (18,19,31). Only 12 (1.7%) of the 722 patients included in the meta-analysis performed by Plonek had significant dilatation of any aortic segment during follow-up and only 13 (1.8%) required reoperation due to a residual or recurrent aortic pathology (30). In this systematic review, only 5 cases of aortic dissection were recorded during the follow-up and only one of those cases was acute. On the other hand, no residual or recurrent aortic pathologies or need for a new correction was recorded in the 272 patients who were only treated with AA wrapping, whereas the combined incidence of these circumstances were 2% and 3% respectively in the 450 patients with concomitant reduction through resection aortoplasty or through plication of the aortic wall. Aortic re-dilatation was observed in areas which were not reinforced or which were insufficiently reinforced, particularly in the noncoronary sinus of Valsalva, and it has been associated with a bad placement or a migration of the prosthesis (34,35). For this reason, Cohen et al. have highlighted the importance of reinforcing this area with a triangular expansion of the aortic wrap, which is anchored to the ventricular-aortic junction (18). In fact, no re-dilatation of the aorta was observed in Plonek’s systematic review whenever external prosthesis was secured with proximal and distal anchorage (30).
Problems/complications
Since wrapping started to be used, there has been concern regarding the possibility that the prosthesis might erode the aortic wall, particularly at both ends, as a consequence of the maintained compression (14). However, this potential complication has not been described in the many series that have analyzed the clinical results, or in the autopsies of patients who died after AA wrapping (18). Bauer et al. described the asymptomatic erosion of the aortic wall underlying a folded area of the external graft found in a patient re-operated because of other causes 4 years after the original operation (34). The formation of folds in the prosthesis might increase the aortic wall stress and promote the formation of a lesion caused by erosion. In order to prevent this, it is important for the prosthesis to be adequately adjusted to the aortic contour. Some authors have hypothesized that the formation of folds is promoted by the corrugated structure of the prosthesis, and recommend positioning the corrugation lines of the graft parallel to the long axis of the aorta to prevent this inconvenient (32).
The so-called “migration” of the prosthesis has been put forward as a potential cause of aortic re-dilatation. It involves the lack of protection and eventual dilatation of areas of the aorta that were initially covered by the wrapping. This event has been associated with the dilatation of the aortic root and it has not been described when the prosthesis was properly anchored to the AA, particularly at the proximal end. This complication has also been related to the transformation of the AA into a cylindrical structure in which the prosthesis migration becomes easier. In order to prevent it, Choi et al. recommend maintaining the fusiform shape of the AA through a slight reduction in the diameter at the proximal and distal segments of the wrapping (32).
Although reoperations are rare in patients who have undergone an AA wrapping, associated or not with other procedures, they cannot be ruled out, particularly in patients with a long life expectancy. The adherence of the vascular prosthesis to the underlying aorta and the surrounding tissues makes reoperation more cumbersome after wrapping than in patients in which the AA has not been previously manipulated. When it is necessary to access the aortic valve in reoperations, it must be done through the vascular prosthesis, and the incision must be sutured en bloc together with the aortic wall when the procedure is finished.
When is AA wrapping indicated?
The most recent clinical practice guidelines recommend replacing the dilated AA in symptomatic patients and in asymptomatic patients with an aortic diameter of 55 mm, except in patients with Marfan syndrome or other connective tissue diseases, in which the required aortic diameter is lesser (36,37). They also recommend AA replacement when the maximal aortic diameter is 45 mm and the patient needs to be operated because a different heart disease, or when concomitant circumstances exists involving a higher risk of dissection or rupture, such as rapid aortic dilatation or a family history of such disorders. In general terms, these guidelines advise against the reduction of the AA diameter through wrapping, although this technique is accepted as an alternative when it is not possible or advisable to use a CPB due to the risks it entails (36).
However, the approach when it is necessary to operate a patient due to a different pathology and the aorta is moderately dilated—between 40 and 50 mm—is still subject to debate. Failing to act on the AA involves the risk that it dilates over time and a new operation may be required, which is an undesirable circumstance. The risk that this happens is particularly high in young patients with a long life expectancy, or in some aortic valve phenotypes which are associated with histological changes in the aortic wall which promote its dilatation, such as bicuspid aortic valve. For this reason, most surgeons prefer to act on the AA in some way. Also, aortic dissections often take place in patients with an aortic diameter lesser than indicated for its replacement, which would justify the adoption of extraordinary measures. The circumstances that need to be taken into consideration when considering wrapping are the size of the aorta, the phenotype of the aortic valve and the AA, the underlying aortic pathology and the estimated risk of the RAA.
Most surgeons who perform wrapping do it in patients with a moderate dilatation of the AA, with a diameter over 40 or 45 mm, depending on the body mass of the patient (11,22). On the other hand, this procedure is not recommended when the AA is significantly dilated (over 55 or 60 mm) due to the risk of fold formation in the aortic wall when the diameter is reduced (11,27,33). However, it is not known whether this factor promotes the appearance of complications or not. When the aorta is highly dilated and conventional AA replacement involves an excessive risk, a reduction aortoplasty should be combined in order to prevent the deformation of the aortic wall.
The most adequate AA phenotype for wrapping is poststenotic fusiform aneurysm, a pathology that represents around 15% of all AA aneurysms (4,18). Other phenotypes, such as annuloaortic ectasia, saccular aneurysms or diffuse tubular dilatation of the entire aorta are not adequate scenarios for wrapping. This technique should also not be used in aortas with a wall showing significant atheromatosis, mural thrombosis, ulcers or calcification.
With regard to the phenotype of the aortic valve, wrapping is specially indicated for patients with BAV in which the concomitant aortopathy promotes a progressive dilatation of the aorta. Some authors advise against wrapping in patients with severe aortic insufficiency due to the higher risk of progressive dilatation of the aortic root, which might promote a distal displacement of the wrapping (19). However, it has been also observed that moderate aortic valve insufficiency disappeared after wrapping in 8 patients with valvular dysfunction due to a dilatation of the STJ (19).
Generally, wrapping is not recommended for patients with Marfan syndrome or other connective tissue diseases (11). However, some teams have used an external aortic root and AA support with a bespoke vascular prosthesis in patients with Marfan syndrome with good initial results (38). Acute or chronic aortic dissection has also been classically considered a contraindication for conservative techniques. In these circumstances, the complete mobilization of the dissected aorta is difficult and risky. Also, theoretically wrapping does not address the main problems of dissection—the intimal tear and the double lumen. In spite of this fact, some teams have used wrapping on patients with Stanford type A acute aortic dissection with quite good results. Lopez et al. treated 6 patients with this technique without having to clamp the aorta and they only required CPB in one case (39). All the patients survived the operation and showed a satisfactory evolution. In the imaging control during follow-up, the false lumen collapsed in 4 patients and thrombosed in 2 other cases. More recently, Demondion et al. have reported the results of AA wrapping without CPB in 15 high-risk patients, comparing them with patients treated with a conventional approach (40). The early mortality of high-risk patients was similar in patients who underwent wrapping and AA replacement (6.7% vs. 11.7%), and both groups also showed similar results regarding their stay in the Postoperative Care Unit and hospital stay. The diameter of the reinforced aorta remained stable during the follow-up period and the false lumen inside the reinforced segment collapsed in all the patients in which the entry tear was located on the AA. However, in one patient with the entry tear located in the aortic arch required the placement of a stent on the AA due to the persistence of the false lumen, and four other patients required endovascular procedures to correct the appearance of ischemia in different territories. According to these experiences, wrapping of the dissected AA may be an acceptable therapeutic choice in patients at very high risk if there is no root dissection, significant aortic insufficiency or organ malperfusion.
All possible contraindications for wrapping of the AA may be disregarded in patients with important comorbidities in which the risk of conventional aortic replacement is too high.
Conclusions
Wrapping of a moderately dilated AA (40 to 55 mm) immediately causes a reverse remodeling of the AA and, to a lower extent, of the neighboring aortic segments. When it is used during the early stages of the disease, before the aorta has undergone irreversible changes, it may normalize its size and the aortic flow, and stop the progression of the disease. Biomechanical studies have demonstrated that this technique reduces the wall stress of the dilated aorta and also the risk of rupture or dissection. Although this procedure modifies the histologic structure of the aortic wall, this does not seem to involve a higher risk of acute aortic complications. This technique may be useful in patients who need to undergo aortic valve surgery or other cardiac procedures without increasing the risk of surgery and with lower morbidity and mortality rates than those of aortic replacement. In any case, it is necessary to carry out an adequate selection of the patients and use a strict surgical technique in order to prevent possible complications. Elastic and easily manageable prostheses should be developed and targeted specifically for wrapping the AA without the drawbacks of conventional vascular prostheses.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Coselli JS, Greenn SY. A brief story of aortic surgery: Insight into distal aortic repair. J Thorac Cardiovasc Surg 2013;145:S123-5. [Crossref] [PubMed]
- Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol 2012;60:1156-62. [Crossref] [PubMed]
- Zierer A, Melby SJ, Lubahn J, et al. Elective surgery for thoracic aortic aneurysms: late functional status and quality of life. Ann Thorac Surg 2006;82:573-8. [Crossref] [PubMed]
- Carrel T, von Segesser L, Jenni R, et al. Dealing with dilated ascending aorta during aortic valve replacement: advantages of conservative surgical approach. Eur J Cardiothorac Surg 1991;5:137-143. [Crossref] [PubMed]
- Matsuyama K, Usui A, Akita T, et al. Natural history of a dilated ascending aorta after aortic valve replacement. Circ J 2005;69:392-6. [Crossref] [PubMed]
- Rylski B, Blanke P, Beyersdorf F, et al. How does the ascending aorta geometry change when it dissects? J Am Coll Cardiol 2014;63:1311-9. [Crossref] [PubMed]
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27. [Crossref] [PubMed]
- Gaudino M, Anselmi A, Morelli M, et al. Aortic expansion rate in patients with dilated post-stenotic ascending aorta submitted only to aortic valve replacement long-term follow-up. J Am Coll Cardiol 2011;58:581-4. [Crossref] [PubMed]
- Lee SH, Kim JB, Kim DH, et al. Management of dilated ascending aorta during aortic valve replacement: Valve replacement alone versus aorta wrapping versus aorta replacement. J Thorac Cardiovasc Surg 2013;146:802-9. [Crossref] [PubMed]
- Robicsek F. A new method to treat fusiform aneurysms of the ascending aorta associated with aortic valve disease: an alternative to radical resection. Ann Thorac Surg 1982;34:92-4. [Crossref] [PubMed]
- Gill M, Dunning J. Is reduction aortoplasty (with or without external wrap) an acceptable alternative to replacement of the dilated ascending aorta? Interact Cardiovasc Thorac Surg 2009;9:693-7. [Crossref] [PubMed]
- Bauer M, Pasic M, Schaffarzyk R, et al. Reduction aortoplasty for dilatation of the ascending aorta in patients with bicuspid aortic valve. Ann Thorac Surg 2002;73:720-3; discussion 724. [Crossref]
- Kiessling AH, Odwody E, Miskovic A, et al. Midterm follow up in patients with reduction ascending aortoplasty. J Cardiothorac Surg 2014;9:120-5. [Crossref] [PubMed]
- Neri E, Massetti M, Tanganelli P, et al. Is it only a mechanical matter? Histologic modifications of the aorta underlying external banding. J Thorac Cardiovasc Surg 1999;118:1116-8. [Crossref] [PubMed]
- Robicsek F, Cook JE, Reames MK, et al. Size reduction ascending aortoplasty: Is it dead or alive? J Thorac Cardiovasc Surg 2004;128:562-70. [Crossref] [PubMed]
- Egloff L, Rothlin M, Kugelmeier J, et al. The ascending aortic aneurysm: replacement or repair? Ann Thorac Surg 1982;34:117-24. [Crossref] [PubMed]
- Treasure T. A. ‘compare and contrast’ exercise: wrapping versus personalised external aortic root support (PEARS). J Cardiothorac Surg 2016;11:104-5. [Crossref] [PubMed]
- Cohen O, Odim J, De la Zerda D, et al. Long-term experience of girdling the ascending aorta with Dacron mesh as definitive treatment for aneurysmal dilation. Ann Thorac Surg 2007;83:S780-4. [Crossref] [PubMed]
- Pecoraro F, Shingaki M, Steuer J, et al. Treatment of isolated ascending aortic aneurysm by off-pump epiaortic wrapping is safe and durable. Interact Cardiovasc Thorac Surg 2016;23:286-291. [Crossref] [PubMed]
- Arsan S, Akgun S, Kurtoglu N, et al. Reduction aortoplasty and external wrapping for moderately sized tubular ascending aortic aneurysm with concomitant operations. Ann Thorac Surg 2004;78:858-61. [Crossref] [PubMed]
- Tappainer E, Fiorani V, Nocchi A, et al. Safe wrapping of the borderline dilated ascending aorta during aortic valve replacement. J Cardiothorac Surg 2007;2:15-22. [Crossref] [PubMed]
- Ang KL, Raheel F, Bajaj A, et al. Early impact of aortic wrapping on patients undergoing aortic valve replacement with mild to moderate ascending aorta dilatation. J Cardiothorac Surg 2010;5:58-61. [Crossref] [PubMed]
- Park JY, Shin JK, Chung JW, et al. Short-term outcomes of aortic wrapping for mild to moderate ascending aorta dilatation in patients undergoing cardiac surgery. Korean J Thorac Cardiovasc Surg 2012;45:148-54. [Crossref] [PubMed]
- Płonek T, Dumanski A. Computed tomography angiography of aorta subjected to external wrapping. J Cardiothorac Surg 2016;11:89-93. [Crossref] [PubMed]
- Tremblay D, Leask RL. Remodelling and pathology development associated with aneurysmal ascending aortic tissues. Can J Chem Eng 2011;89:13-22. [Crossref]
- Schiffrin EL. Vascular Stiffening and Arterial Compliance. Implications for Systolic Blood Pressure. Am J Hypertens 2004;17:39S-48S. [Crossref] [PubMed]
- Plonek T, Rylski B, Dumanski A, et al. Biomechanical analysis of wrapping of the moderately dilated ascending aorta. J Cardiothorac Surg 2015;10:106-11. [Crossref] [PubMed]
- Giudici F, Qian Y, O’Rourke M, et al. Simulation of reduction of proximal aortic stiffness by an elastic wrap and effects on pulse pressure. Conf Proc IEEE Eng Med Biol Soc 2012;2012:657-60.
- Doyle M, Peeceeyan S, Bonar F, et al. Rarefaction of the aorta under dacron wrap: A rare complication. Interact Cardiovasc Thorac Surg 2014;19:341-3. [Crossref] [PubMed]
- Plonek T. A Metaanalysis and systematic review of wrapping of the ascending aorta. J Card Surg 2014;29:809-15. [Crossref] [PubMed]
- Plonek T, Dumanski A, Nowicki R, et al. Single center experience with wrapping of the dilated ascending aorta. J Cardiothorac Surg 2015;10:168-71. [Crossref] [PubMed]
- Choi MS, Jeong DS, Lee HL, et al. Aortic wrapping for a dilated ascending aorta in bicuspid aortic stenosis. Circ J 2015;79:778-84. [Crossref] [PubMed]
- Zhang H, Lu F, Qu D, et al. Treatment of fusiform ascending aortic aneurysms: A comparative study with 2 options. J Thorac Cardiovasc Surg 2011;141:738-43. [Crossref] [PubMed]
- Bauer M, Grauhan O, Hetzer R. Dislocated wrap after previous reduction aortoplasty causes erosion of the ascending aorta. Ann Thorac Surg 2003;75:583-4. [Crossref] [PubMed]
- Akgun S, Atalan N, Fazliogullari O, et al. Aortic root aneurysm after off-pump reduction aortoplasty. Ann Thorac Surg 2010;90:e69-70. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the european society of cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Hiratzka LF, Creaguer MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves. A statement of clarification from the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016;133:680-6. [Crossref] [PubMed]
- Pepper J, John Chan K, Gavino J, et al. External aortic root support for Marfan syndrome: Early clinical results in the first 20 recipients with a bespoke implant. J R Soc Med 2010;103:370-5. [Crossref] [PubMed]
- Lopez S, Roux D, Cazavet A, et al. Wrapping procedure for Stanford type A acute aortic dissection: Is there an indication for surgery without a cardiopulmonary bypass? Ann Thorac Surg 2012;94:990-1. [Crossref] [PubMed]
- Demondion P, Ramadan R, Azmoun A, et al. Aortic Wrapping for Stanford Type A Acute Aortic Dissection: Short and Midterm Outcome. Ann Thorac Surg 2014;97:1590-6. [PubMed]


