Benefits of postoperative thoracic radiotherapy for small cell lung cancer subdivided by lymph node stage: a systematic review and meta-analysis
Introduction
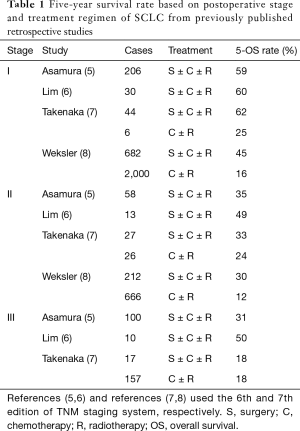
The most common cause of cancer-related deaths worldwide is lung cancer, among which small cell lung cancer (SCLC) accounts for approximately 15% (1). SCLC is extremely malignant and characterized by a rapidly progressing primary mass, early lymph node metastases and distant disseminations (2). As recommended in the European Society for Medical Oncology (ESMO) (3) and National Comprehensive Cancer Network (NCCN) (4) guidelines, the combination of chemotherapy and thoracic radiotherapy is the current standard of care for limited-stage SCLC, and subsequent prophylactic cranial irradiation (PCI) is given when patients show significant response to chemoradiotherapy. The progress made in early diagnosis and surgical techniques for lung cancer in recent years has resulted in surgery having a more important role in limited early stage SCLC. Some retrospective analyses have shown that surgical-based multi-modality treatment significantly improved the 5-year survival compared with chemoradiotherapy alone for patients with early SCLC (5-9) (summarized in Table 1). Thus, in fit patients, especially those with peripherally located and early T stage SCLC, and negative mediastinal lymph nodes on CT scan, PET-CT scan or EBUS and/or mediastinoscopy, surgery may be a valid alternative to chemoradiotherapy (3,4). However, the local-regional recurrence (LRR) rate still ranged from 10–20% after surgery (10,11), underscoring the need to define better postoperative therapeutic strategies.
Full table
Recently, a population-based analysis of the National Cancer Database showed that for patients who underwent complete resection and had postoperative pT1-2N0M0 SCLC, adjuvant chemotherapy with or without PCI was associated with improved survival compared with those without adjuvant therapy. The median overall survival (OS) and 5-year survival rates were 66 months and 53%, respectively (12). It has been recognized that thoracic postoperative radiotherapy (PORT) cannot further improve survival for non-SCLC (NSCLC) patients who received complete resection and presented with postoperative pN0- or pN1-staged disease (13). However, it has been controversial whether PORT could reduce the recurrence risk and improve survival for patients with different stages of metastatic lymph node (pN0, pN1, and pN2) SCLC. Therefore, a meta-analysis was performed to evaluate the benefit of PORT for patients with different postoperative lymph node-staged SCLC.
Methods
Search strategy and study selection
Search strategy
An electronic search of the PubMed, OVID, Web of SCI, EMBASE, Google Scholar, Cochrane Library, Chinese National Knowledge Infrastructure and Wanfang databases were performed on February 29, 2016, using the following retrieval details: (small cell lung cancer [Title] NOT non-small [Title]) AND ((post-operative OR postoperative OR surgery) AND (radiotherapy OR radiation therapy) OR PORT). The published languages and years were not limited. The computer search was supplemented with manual searches of the listed references in all retrieved articles, primary studies and abstracts from meetings, such as American Society of Clinical Oncology (ASCO), ESMO and World Conference of Lung Cancer (WCLC).
Inclusion criteria
Studies were included if they met the following inclusion criteria: (I) research type: prospective or retrospective cohort study; (II) patients: the criteria for eligible patients included histologically or cytologically confirmed SCLC; patients with SCLC underwent radical surgery; an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; (III) intervention measure: compared PORT with non-PORT according to postoperative lymph node stage; (IV) research outcome: reported survival (DFS and/or OS) and/or relapse data, regardless of the publication status (published, conference proceedings, or unpublished).
Exclusion criteria
Studies were excluded if they met any of the following exclusion criteria: (I) low sample size (n<10); (II) systematic reviews and repeated published studies; if similar papers were published by the same organization, then we included the most comprehensive study; (III) survival and/or relapse data could not be extracted from the literature.
Study screening and data extraction
Two investigators (Zhang SL and Sun X) independently inspected each reference and applied the inclusion criteria. For possibly relevant articles or in cases of disagreement, both investigators inspected the full text independently, and the inclusion/exclusion of conflicting studies was decided by consultation with a third investigator. A standardized approach was used to extract data from each article such as publication details, quality scores, first author’s name, year of publication, case number and lymph node stage. Each publication was carefully examined, including the names of all authors, to avoid duplication of data. We also extracted the outcome measures of each study as follows: the 1-, 3- and 5-year OS rates and/or the 1-, 2- and 3-year LRR rates from the PORT and non-PORT groups, as well as the OS and/or LRR rates of N0-, N1- and N2-staged patients from the two groups separately. As some studies did not report the OS rate directly, Kaplan-Meier curves were read using Engauge Digitizer software version 4.1 (http://sourceforge.net).
Quality assessment and statistical analysis
Quality assessment
The studies in the meta-analyses were assessed based on the 9-star Newcastle-Ottawa Scale for quality (non-randomized). The tool was used to assess risk of bias, including representativeness, selection of the non-exposed cohort, ascertainment of exposure, demonstration, assessment of outcome, follow-up and adequacy of follow up.
Statistical analysis
Data were analyzed using STATA 12.0 (STATA Corp, College Station, TX, USA) statistical software. As the median survival and HR in partial studies could not be obtained or extracted from the original papers, we calculated the relative ratio (RR) with a 95% confidence interval (CI) instead of HR using STATA 12.0 to analyze the 1-, 3-, and 5-year OS rates and the 1-, 2-, and 3-year LRR rates between the PORT and non-PORT group, using a previously published method (14). A P value less than 0.05 was considered statistically significant. The inconsistency index (I2 statistic) was used to assess heterogeneity among the studies. If P<0.10 and I2 >50%, a random-effects model was usually used to examine the reason for the heterogeneity. Otherwise, the fixed-effects model was used.
Results
Retrieved literature
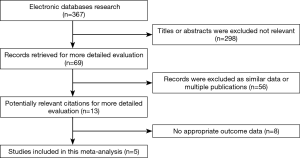
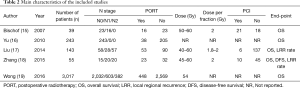
A total of 367 relevant studies were retrieved according to the outlined strategy. Based on the inclusion and exclusion criteria, 362 studies were excluded by reading the title, abstract and full text. Finally, five studies, including 3,497 patients, were included for the meta-analysis (15-19), and all were retrospective cohort studies. The PORT dose patients received between 40 and 60 Gy using 1.8–2.0 Gy/fraction (15,17-19), and was not reported in one study (16); and partial patients received PCI (Table 2). The T stage in 3,497 patients was mainly T1-2. There were 3,142 T1-2 stage patients, 115 T3 stage patients, and 97 T4 stage patients. We could not extract the T stage details of the remaining 143 patients. A flow chart depicting the strategy is shown in Figure 1.
Full table
Quality assessment of the included studies
All trials were assessed using the Newcastle-Ottawa Scale, with more stars indicating better quality. The studies included in this analysis scored 6–7 (Table 3).

Full table
Comparing survival rates between the PORT and non-PORT groups
All patients
Heterogeneity was found after applying the heterogeneity test (I2 =70.8% for the 3-OS rate), thus a random effect model was used for OS rate analysis. The results showed that PORT did not improve the 1-, 3- or 5-year OS rates when all patients (n=3,497) were analyzed together (RR =0.66, 0.81 and 0.99; P=0.135, 0.251 and 0.926, respectively) (Figure S1A).
Subgroup analysis based on lymph node stage
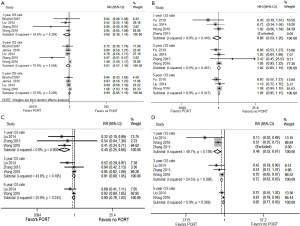
No heterogeneity was found in the comparisons made according to different lymph node stage. In pN0 patients (n=2,371), no significant difference was seen in 1-, 3- or 5-year OS rates between the PORT and non-PORT groups (RR =0.86, 1.02 and 1.05; P=0.447, 0.753 and 0.337, respectively) (Figure S1B).
In pN1 patients (n=667), no significant difference was seen in the 3- or 5-year OS rates between the PORT and non-PORT groups (RR =0.91 and 0.90, P=0.207 and 0.114, respectively); however, an improved 1-year survival rate was found in the PORT group compared with the non-PORT group (RR =0.40; P<0.001) (Figure S1C).
In pN2 patients (n=459), the results showed that PORT significantly improved 1-, 3- and 5-year OS rates (RR =0.46, 0.72 and 0.85; P<0.001, <0.001 and P=0.005, respectively) (Figure S1D).
Comparing LRR rates between the PORT and non-PORT groups
All patients
No heterogeneity was found between the studies using the heterogeneity test. The results showed that PORT significantly reduced 1-, 2- and 3-year LRR rates for SCLC patients (RR =0.14, 0.28 and 0.27; P=0.002, 0.001 and P<0.001, respectively) (Figure S2A).
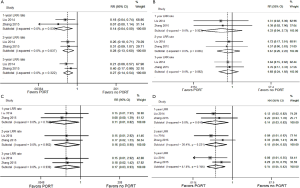
Subgroup analysis based on lymph node stage
No heterogeneity was found in the comparisons made according to different lymph node stage. Subgroup analyses showed that in pN0 patients, there was no statistical difference in 1-, 2- and 3-year LRR rates between the PORT and non-PORT groups (RR =0.33, 0.67 and 0.68; P=0.305, 0.514 and 0.471, respectively) (Figure S2B). However, in pN1 and pN2 patients, PORT significantly reduced 1-, 2- and 3-year LRR rates (RR =0.11, 0.16 and 0.17; P=0.032, 0.037 and 0.041, respectively for pN1 patients; RR =0.14, 0.15 and 0.15; P=0.009, 0.003 and P<0.001, respectively for pN2 patients) (Figure S2C,D).
Discussion
Whether additional thoracic PORT is beneficial for patients with limited stage SCLC who have received radical operations and subsequent chemotherapy remains controversial. Although members of the NCCN Committee agreed that SCLC patients with pathologically confirmed lymph node metastases should be treated with PORT, this recommendation was only based on lower-level evidence and was not supported by prospective data. This is the first meta-analysis to analyze the benefits of PORT on limited stage SCLC patients, despite the fact that the pooled data were based on retrospective studies. Five retrospective cohort studies with a total of 3,497 cases were included in this analysis. The results demonstrated that for pN2-SCLC patients, PORT significantly reduced the 1-, 2- and 3-year LRR rates, and simultaneously increased the 1-, 3- and 5-year OS rates; for pN1-SCLC patients, PORT also significantly reduced the 1-, 2- and 3-year LRR rates, and increased the 1-year OS rate, but failed to improve the 3- and 5-year OS rates; for pN0-SCLC patients, PORT had no clinical benefit on either OS or LRR rates. Our meta-analysis suggested that PORT should be conventionally performed in patients with postoperative lymph node positive SCLC, although the long-term survival benefit of PORT in pN1-SCLC patients requires further study.
Several series (7,8,20-24) have reported good outcomes in patients with limited early stage SCLC who received surgical-based multi-modality treatments. Therefore, patients with limited early stage SCLC with clinical T1-2N0-1M0 may be considered for lobectomy plus systematic mediastinal lymphadenectomy (3,4). Moreover, different surgical approaches might have different effects on survival. A study which included 28,621 cases of stage I-IIIA SCLC from the National Cancer Database (25) showed that 5-year survival rates were significantly improved in patients who underwent lobectomy compared with those who underwent segmentectomy or pneumonectomy (40% vs. 21% vs. 22%, respectively). In addition, sublobar resection for early SCLC was obviously associated with increased risk of death compared with lobectomy in the Cox Proportional Hazards analysis of mortality when controlling for patient and tumor characteristics. Additionally, the 5-year survival could be further prolonged in patients receiving postoperative chemotherapy compared with those receiving surgery alone, thus postoperative adjuvant chemotherapy has been the standard of care for patients with limited early stage SCLC who underwent radical surgery due to the improvement in long-term survival.
Nevertheless, due to the high recurrence risk and poor prognosis after surgery, the introduction of PORT may further improve prognosis for patients with limited stage SCLC. Zhou et al. (26) reported a cohort study that showed PORT could improve survival in patients with SCLC; however, Varlotto et al. (27) found that patients with limited early stage (stage I–II) SCLC did not benefit from PORT, and segmentectomy was inferior to lobectomy but superior to radiotherapy alone. Zhu et al. (28) proposed that PORT should be given for patients with primary lesions greater than 5 cm or postoperative positive lymph nodes. The abovementioned reports mainly involved patients with postoperative stage I–III, but did not further explore a correlation between survival and lymph node status and treatment factors. Recently, there were a few studies that tested PORT on different lymph node-staged SCLC. These retrospective studies showed that PORT reduced the local recurrence rate and simultaneously improved the survival rate in patients with postoperative lymph node positive, especially pN2-staged, SCLC (17-19,29). However, the cohort size in each study was relatively small. This meta-analysis provided a large number of pooled patients from clinical practices; however, it must be noted that only two of the included studies analyzed the 5-year survival rate in patients with pN1 disease. Thus, interpretation of the long-term survival effect of PORT in pN1-SCLC patients should be carried out with caution. In addition, despite the lack of detailed information regarding T stage, the present meta-analysis did not further analyze the effect of T stage on the benefit of PORT for SCLC subdivided by lymph node stage, as patients mainly had T1-2 stage. Currently, the optimal radiation dose and fractions of PORT for SCLC have not been established. Based on the studies included in this meta-analysis, commonly used prescription doses and fractions were 40–60 Gy delivered in 1.8–2.0 Gy/fraction.
There are some limitations in our meta-analysis. Firstly, all included studies were retrospective studies, and the quality assessment showed low scores of grades 6–7, leading to relatively unstable reliability of the results; additionally, due to the limited number of studies included (n=5), we did not draw an Egger funnel plot to detect bias or analyze sensitivity to ensure the stability of the conclusions of this meta-analysis. Secondly, for the meta-analysis based on time to event studies, pooling the HR is preferred, as few studies reported PORT-related survival and LRR. Only five studies were included, the median survival and HR in partial studies could not be extracted; therefore, we calculated the RR based on the data of the 1-, 3-, and 5-year OS rates and the 1-, 2-, and 3-year LRR rates extracted from the included studies, although the extracted data may not be consistent with the original data. Thirdly, some confounding factors may have affected the OS, such as differences in institutions, operation types, dose of PORT, PCI, and cycles of chemotherapy. For example, due to the absolute benefit of PCI on long-term survival, at present, PCI is mainly recommended for patients who have undergone a radical resection and completed adjuvant chemotherapy (30,31). In the included studies, PCI was only given to some patients; therefore, PCI may be a confounding factor for survival; however, we could not extract accurate and detailed information on PCI between the PORT and non-PORT group. Therefore, a prospective randomized clinical trial is needed to validate these results.
In conclusion, although derived from retrospective cohort studies, the present study demonstrates that PORT is beneficial in pN2 patients as it improves long-term survival and reduces local regional recurrences (LRRs), and improves 1-year survival rate and reduces LRRs in pN1 patients. However, the current evidence does not support any benefit in using PORT in terms of recurrence risk or survival, in patients with postoperative pN0 SCLC. Due to quality limitations in the studies included in this meta-analysis, further well-designed, prospective studies are needed to further confirm these findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Oberg K, Hellman P, Kwekkeboom D, et al. Neuroendocrine bronchial and thymic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v220-2. [Crossref] [PubMed]
- Goldberg SB, Willers H, Heist RS. Multidisciplinary management of small cell lung cancer. Surg Oncol Clin N Am 2013;22:329-43. [Crossref] [PubMed]
- Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi99-105. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Small Cell Lung Cancer, Version 2. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Asamura H, Goya T, Koshiishi Y, et al. A Japanese Lung Cancer Registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol 2008;3:46-52. [Crossref] [PubMed]
- Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267-71. [Crossref] [PubMed]
- Takenaka T, Takenoyama M, Inamasu E, et al. Role of surgical resection for patients with limited disease-small cell lung cancer. Lung cancer 2015;88:52-6. [Crossref] [PubMed]
- Weksler B, Nason KS, Shende M, et al. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg 2012;94:889-93. [Crossref] [PubMed]
- Zhu H, Zhou Z, Xue Q, et al. Treatment modality selection and prognosis of early stage small cell lung cancer: retrospective analysis from a single cancer institute. Eur J Cancer Care (Engl) 2013;22:789-96. [Crossref] [PubMed]
- Rea F, Callegaro D, Favaretto A, et al. Long term results of surgery and chemotherapy in small cell lung cancer. Eur J Cardiothorac Surg 1998;14:398-402. [Crossref] [PubMed]
- Tsuchiya R, Suzuki K, Ichinose Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg 2005;129:977-83. [Crossref] [PubMed]
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Fan C, Gao S, Hui Z, et al. Risk factors for locoregional recurrence in patients with resected N1 non-small cell lung cancer: a retrospective study to identify patterns of failure and implications for adjuvant radiotherapy. Radiat Oncol 2013;8:286. [Crossref] [PubMed]
- Zhang H, Zhou Y, Yuan G, et al. Antiviral therapy improves the survival rate and decreases recurrences and fatalities in liver cancer patients following curative resection: A meta-analysis. Mol Clin Oncol 2015;3:1239-47. [PubMed]
- Bischof M, Debus J, Herfarth K, et al. Surgery and chemotherapy for small cell lung cancer in stages I-II with or without radiotherapy. Strahlenther Onkol 2007;183:679-84. [Crossref] [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [Crossref] [PubMed]
- Liu WS, Zhao LJ, Wang S, et al. Benefits of postoperative radiotherapy in multimodality treatment of resected small-cell lung cancer with lymph node metastasis. Eur J Surg Oncol 2014;40:1156-62. [Crossref] [PubMed]
- Zhang SL, Han CB. Clinical significance of postoperative radiotherapy in small cell lung cancer patients with different lymph node staging after surgery. Chinese Journal of Clinical Research 2015;28:689-92.
- Wong AT, Rineer J, Schwartz D, et al. Assessing the Impact of Postoperative Radiation Therapy for Completely Resected Limited-Stage Small Cell Lung Cancer Using the National Cancer Database. J Thorac Oncol 2016;11:242-8. [Crossref] [PubMed]
- Lüchtenborg M, Riaz SP, Lim E, et al. Survival of patients with small cell lung cancer undergoing lung resection in England, 1998-2009. Thorax 2014;69:269-73. [Crossref] [PubMed]
- Kawano D, Okamoto T, Fujishita T, et al. Surgical results of resectable small cell lung cancer. Thorac Cancer 2015;6:141-5. [Crossref] [PubMed]
- Takei H, Kondo H, Miyaoka E, et al. Surgery for small cell lung cancer: a retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2014;9:1140-5. [Crossref] [PubMed]
- de Hoyos A, DeCamp MM. Surgery for small cell lung cancer. Thorac Surg Clin 2014;24:399-409. [Crossref] [PubMed]
- Clemente M, Soccal PM, Rochat T, et al. The role of surgery in early stage small cell lung cancer: should it be re-evaluated?. Rev Med Suisse 2013;9:2170-2172-4. [PubMed]
- Combs SE, Hancock JG, Boffa DJ, et al. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J Thorac Oncol 2015;10:316-23. [Crossref] [PubMed]
- Zhou Z, Wang L, He J, et al. Clinical study of adjuvant chemoradiotherapy after surgery for patients with limited disease-small cell lung cancer. Paper presented at: The sixth annual meeting of China Society for Radiation Oncology 2007; Beijing, China.
- Varlotto JM, Recht A, Flickinger JC, et al. Lobectomy leads to optimal survival in early-stage small cell lung cancer: a retrospective analysis. J Thorac Cardiovasc Surg 2011;142:538-46. [Crossref] [PubMed]
- Zhu XM. For patients with limited disease-small cell lung cancer after surgery: radiation therapy or not? Paper presented at: The sixth annual meeting of China Society for Radiation Oncology 2007; Beijing, China.
- Schreiber D, Rineer J, Weedon J, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer 2010;116:1350-7. [Crossref] [PubMed]
- Le Péchoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy. Lancet Oncol 2009;10:467-74. [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]