Identifying patients at higher risk of pneumonia after lung resection
Introduction
Lung cancer is the most common cause of cancer-related deaths worldwide (1). Surgical resection offers the best part in the treatment for lung cancer. However, the patients undergoing lung cancer surgery are susceptible to pulmonary complications because of pain, secretory retention, inefficient cough, and decreased immunity. Postoperative pneumonia (POP) is one of the most frequent complications following thoracic surgery and is strongly associated with high mortality rates (2,3). Many perioperative risk factors have been reported to be associated with an increased incidence of pneumonia after surgery (2,4-8). However, there was no specific scoring system constructed to date for the prediction of POP after lung resection. Therefore, we wanted to re-identify the risk factors of pneumonia after lung cancer surgery and develop a scoring system to stratify patients with increased risk of POP.
Methods
Patients selection
After approval from the ethics board, a multi-center prospective registry study from six hospitals in China was conducted. Five hundred patients from September 2014 to June 2016 were registered under the impression of primary lung cancer. Among them, we excluded 31 patients who underwent wedge resection and 3 patients who were diagnosed with stage IV non-small cell lung cancer (NSCLC). According to the study’s exclusion criteria, patients with interstitial pneumonitis, pulmonary fibrosis, severe pulmonary emphysema, prior chemotherapy or radiation therapy for malignant diseases, and previous ipsilateral thoracotomy were excluded before enrolling into this study. Finally, a total of 466 patients were included in the study.
Perioperative management to reduce POP
Pneumonia is a major cause of postoperative mortality and thus, it is important to prevent it from happening. Smoking cessation helps to decrease sputum production and improves pulmonary function and immune mechanisms in the long-term. Good nutritional status is necessary for wound healing after surgery and beneficial in decreasing the complications. Deep breathing exercises improve spirometry values. Assisted coughing helps expectoration easy. Atomization inspiration helps to dilate airways and loosen the secretions. Early mobilization facilitates airway clearance and reduces the risk of immobility associated with postoperative pulmonary complication such as pneumonia, atelectasis and pulmonary embolism. All patients received postoperative prophylactic antibiotics. Pain management plays an important role in the reduction of pulmonary complications following thoracic surgery. Two of the most common methods for postoperative pain relief are intercostal nerve block analgesia at the end of the operation and patient-controlled analgesia (PCA). These methods help to alleviate muscle pain triggered by coughing or other circumstances.
Diagnosis of POP
The diagnosis of suspected pneumonia includes three criteria which were as follows: temperature >38.0 °C, white cell count greater than 10×109/L and chest X-ray plain film findings of consolidation. CT-scan was used in all cases to establish a more “anatomical” form of the suspicion of POP based on postoperative fever, leukocytosis etc. Sputum cultures and blood cultures were performed as necessary.
Data collection
Data were collected on basic demographics, which included age, gender, body mass index (BMI), smoking (current or previous smoker vs. never smoker), comorbid conditions (CCI score), percent forced expiratory volume in one second (FEV1%), operative approach [video-assisted thoracoscopic surgery (VATS) vs. converted open], extent of excision, operation time, pathological stage of disease.
Statistical analysis
Results were expressed as mean ± SD for continuous variables and as count and proportions for categorical variables. For univariate analysis of risk factors, Student’s t-tests were used for continuous data, and Pearson’s chi-squared or Fisher’s exact test were used for categorical data. All P values were two-sided and results were considered significant if P<0.05. Significant variables associated with POP were evaluated using stepwise logistic regression analysis. Continuous and ordinal variables were dichotomized. Discrimination of the model was assessed with the area under receiver operating characteristic (ROC). Calibration of the model was evaluated with the Hosmer-Lemeshow (H-L) goodness-of-fit test. Internal validation was assessed using the 1,000 bootstrap resamples method (9). A ROC curve was made to identify the optimal cutoff point that represents the best compromise between sensitivity and specificity. The data was calculated using IBM SPSS software package version 22.0 and R for Mac OS X version 3.3.2.
Results
From September 2014 to June 2016, a total of 466 patients who met the inclusion criteria were enrolled in the study. POP occurred in 17 patients (3.6%). There were 237 males and 229 females patients. One hundred and fifty-nine patients (34.1%) had smoking history. The mean ± SD age of the group was 61±10 years, mean BMI was 24±3 kg/m2, mean FEV1% was 79%±10%, and mean operation time was 168±63 mins. One hundred and fifty-four patients (33.0%) had a CCI score greater than 1. There were 458 patients (98.3%) who underwent VATS completely and 8 patients (1.7%) had converted to open lobectomy. The most frequent procedure was lobectomy (n=447, 95.9%) followed by bilobectomy (n=10, 2.1%) and sleeve resection (n=5, 1.1%). The least frequent procedure was pneumonectomy (n=4, 0.9%). Majority of patients had pathological stage I disease (n=373, 80%) followed by stage III disease (n=73, 15.7%) and stage II disease (n=20, 4.3%). The characteristics of the patients in the study were shown in Table 1.
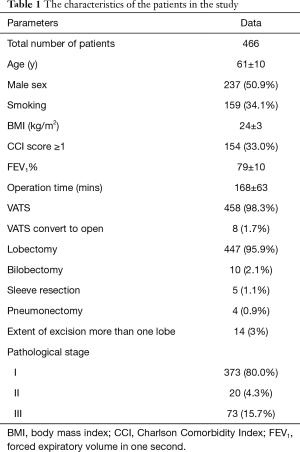
Full table
Univariate analysis showed age, smoking, pathological stage and extent of excision as significant variables associated with POP after lung resection (Table 2), while gender, BMI, comorbid conditions, FEV1%, operation time, or operative approach were not considered as significant variables.
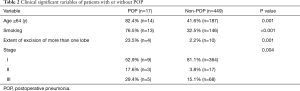
Full table
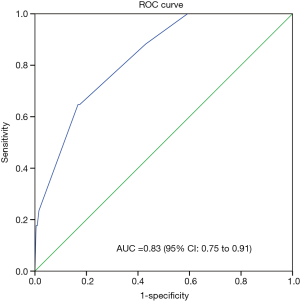
Perioperative variables associated with POP were then identified using stepwise logistic regression analysis. Three independent risk factors were associated with the occurrence of POP: age ≥64 years, smoking, and extent of excision of more than one lobe (Table 3). Since multicollinearity problems lead to biased estimation and affects the efficiency of a predictive model, we need to confirm that the predictor variables in the model are independent one another. Condition Index (CI) and variance proportions (VP) were employed to check for multicollinearity. The maximum value of CI was 18.36, and corresponding VP values of age and stage were 60% and 52%, respectively. These results suggested the multicollinearity problem associated with age and stage, and also explained as to why the stage didn’t appear to be significant in stepwise logistic regression analysis. Based on the information of the related studies, the pathologic stage seemed not significantly associated with the POP (6,7), while age was strongly associated with the POP (4,6,8,10). Hence, it is reasonable to drop the stage variable from the model (11). The model was developed with the following formula: logit (POP) = −5.54 + 1.80 × age (coded 1 for age ≥64 y) + 1.62 × smoke (coded 1 for having smoke history) + 2.35 × extent of excision (coded 1 for extent of more than one lobe). The value of AUC was 0.830, and the value of H-L statistic was 1.57 (P=0.451). This indicates good discrimination and calibration of the model. Figure 1 shows the predicted probability of POP plotted against actual probability of POP.

Full table

Risk score was developed based on the proportional weighting of the coefficients, and assigned 3 points to the lowest coefficient (smoking): Smoking, 3 points; age ≥64 y, 4 points; extent of excision of more than one lobe, 5 points. The points were summed for each patient to generate an aggregate score. The aggregate scores varied from 0 to 12 points. Patients with scores associated with similar risk of POP were then grouped in four classes of incremental POP risk (Table 4). An aggregate score of 0–5 was associated with a POP of 1.58%, score of 7 with a POP of 9.33%, score of 8–9 with a POP of 16.67%, score of 12 or more with a POP of 60.00%. The risk scoring system showed relatively high predictive ability for POP. The best cut-off score value as determined by Youden’s index was 5 points, which defined the best combination of sensitivity (64.7%) and specificity (83.3%) (Figure 2).
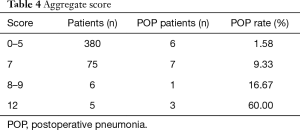
Full table
Discussion
POP is one of the most frequent complications in patients undergoing radical resection of lung cancer, and is very likely to cause respiratory failure, acute respiratory distress syndrome, or even death if untreated in time. POP is also proved to be associated with decreased long-term survival (7). Therefore, it is important to recognize the risk factors associated with POP and reduce the occurrence of POP. We identified three risk factors for POP following lung cancer surgery: age ≥64 years, smoking, and extent of excision of more than one lobe. All of the three risk factors were well corroborated by several other previous studies.
Among the factors we identified, extent of excision seems to be the major predictors of POP. Patients with more than one lobe resection have about 10-fold risk of POP compared to those who underwent only one lobe excision. Yano et al. identified a 3-fold higher risk of morbidity including POP for patients who underwent combined resection (4). It was also confirmed that a reduced FEV1% did was related to the increased respiratory morbidity and mortality (12). Kim et al. found that for preserving pulmonary function after NSCLC surgery, VATS sub-lobar resection outweighed VATS lobectomy, and surgery of the right upper lobe or right middle lobe outweighed other sites because of their relatively small size (13). Therefore, we speculate that the extent of excision has an impact on the pulmonary reserve, which may further influence the incidence of POP.
Age is another important predictor for POP. We found that age ≥64 years was an independent predictor for POP. Similar to our study, Iwamoto et al. found that patients older than 65 years who underwent thoracic surgery were associated with the highest incidence of pneumonia (10). Shiono et al. reported that age older than 75 years was an independent significant risk factor for POP (8). However, the observation of increased POP risk with advanced age showed contrary to the findings to the Schussler et al. study (14). This might be due to the different definitions for advanced age and the number of patients selected.
Smoking as a risk factor for POP had been widely reported. Schussler et al. showed an increased risk for POP in smokers compared to never smokers (OR=9.43) (14). According to a study with 417 lung cancer patients who underwent surgical resection, 92.3% patients had smoking history in the POP group while only 52.8% patients had smoking history in the non-POP group (6). Barrera et al. reported that the risk of pneumonia after anatomical lung resection was significantly lower in nonsmokers (3%) compared to all smokers (11%), and found that the patients with a smoking history of more than 60 pack-years were more likely to develop pulmonary complications (15). It was widely documented that cigarette smoke induces pulmonary inflammation. In a recent review regarding inflammatory diseases of the lung induced by conventional cigarette smoke, cellular and molecular mechanisms of smoke-induced inflammation were listed in great detail (16). It is important to encourage patients to stop smoking prior to surgery as soon as possible.
According to univariate analysis, pathological stage was determined to be a significant independent risk factor for POP. However, statistical significance was not observed (P>0.05) in multivariate analyses. The observation of our study was consistent with the findings of some reports (6,7), while disagreed with the findings of Shiono et al. (8), which confirmed an increased risk of POP for cancer stages ≥III compared to stages I/II. Although statistical significance was not reached, we could still find the proportion of patients with POP in stages II/III to be higher than stages I. More data are needed to further explore the relationship between POP and pathologic stage.
Finally, we constructed a scoring system that could predict the risk of POP with the above-mentioned three risk factors, which can be easily observed by a surgeon. The value of AUC was 0.830, which represented a perfect discrimination to predict the patients at higher risk of POP.
There are several limitations in our report. Firstly, compared to most of the previously reported studies, our observed POP rate was 3.6% which was somewhat lower (3,6,14). This might be due to relatively small scale of this registry study. On the other hand, VATS is a reliable approach for the treatment of lung cancer with low complication rate and has been well confirmed by other studies (17,18). Complete VATS was applied in 98.3% of patients in our study, which to some extent might be the reason for the low incidence rates of POP. Secondly, few previous studies have reported the risk factors for POP but our study showed no significance with these predictors, i.e., BMI, CCI score, FEV1%. This is probably due to the fact that the patients included in this study are highly selected, i.e., with early clinical stage, good pulmonary function, good control of comorbidities, no prior chemotherapy or radiation therapy. Finally, the risk scoring system we constructed need external validation.
In spite of these limitations mentioned above, we still think that the scoring system seems to achieve a good performance. To our knowledge, this is the first study to construct a specific risk scoring system to distinguish and stratify lung cancer patients with higher risk of POP. Moreover, we used only three risk factors, which could be easily recognized by the surgeons.
Conclusions
Patients with older age, smoking and extent of excision of more than one lobe have a higher risk for pneumonia after lung cancer surgery. The proposed risk scoring system is simple, and may help surgeons to effectively identity patients at higher risk for POP and take perioperative interventions as appropriate.
Acknowledgements
Funding: This work was supported by Beijing Municipal Commission of Science and Technology (D141100000214004).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Peking University People’s Hospital (IRB No. 2014PHB033-01) and registered at ClinicalTrials.gov (ID: NCT01707888). Written informed consent was signed by all patients.
References
- Algar FJ, Alvarez A, Salvatierra A, et al. Predicting pulmonary complications after pneumonectomy for lung cancer. Eur J Cardiothorac Surg 2003;23:201-8. [Crossref] [PubMed]
- Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815-8. [Crossref] [PubMed]
- Andalib A, Ramana-Kumar AV, Bartlett G, et al. Influence of postoperative infectious complications on long-term survival of lung cancer patients: a population-based cohort study. J Thorac Oncol 2013;8:554-61. [Crossref] [PubMed]
- Yano T, Yokoyama H, Fukuyama Y, et al. The current status of postoperative complications and risk factors after a pulmonary resection for primary lung cancer. A multivariate analysis. Eur J Cardiothorac Surg 1997;11:445-9. [Crossref] [PubMed]
- Ploeg AJ, Kappetein AP, van Tongeren RB, et al. Factors associated with perioperative complications and long-term results after pulmonary resection for primary carcinoma of the lung. Eur J Cardiothorac Surg 2003;23:26-9. [Crossref] [PubMed]
- Lee JY, Jin SM, Lee CH, et al. Risk factors of postoperative pneumonia after lung cancer surgery. J Korean Med Sci 2011;26:979-84. [Crossref] [PubMed]
- Simonsen DF, Sogaard M, Bozi I, et al. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med 2015;109:1340-6. [Crossref] [PubMed]
- Shiono S, Yoshida J, Nishimura M, et al. Risk factors of postoperative respiratory infections in lung cancer surgery. J Thorac Oncol 2007;2:34-8. [Crossref] [PubMed]
- Brunelli A, Rocco G. Internal validation of risk models in lung resection surgery: bootstrap versus training-and-test sampling. J Thorac Cardiovasc Surg 2006;131:1243-7. [Crossref] [PubMed]
- Iwamoto K, Ichiyama S, Shimokata K, et al. Postoperative pneumonia in elderly patients: incidence and mortality in comparison with younger patients. Intern Med 1993;32:274-7. [Crossref] [PubMed]
- Greenland S, Daniel R, Pearce N. Outcome modelling strategies in epidemiology: traditional methods and basic alternatives. Int J Epidemiol 2016;45:565-75. [Crossref] [PubMed]
- Magdeleinat P, Seguin A, Alifano M, et al. Early and long-term results of lung resection for non-small-cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg 2005;27:1099-105. [Crossref] [PubMed]
- Kim SJ, Ahn S, Lee YJ, et al. Factors associated with preserved pulmonary function in non-small-cell lung cancer patients after video-assisted thoracic surgery. Eur J Cardiothorac Surg 2016;49:1084-90. [Crossref] [PubMed]
- Schussler O, Alifano M, Dermine H, et al. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med 2006;173:1161-9. [Crossref] [PubMed]
- Barrera R, Shi W, Amar D, et al. Smoking and timing of cessation: impact on pulmonary complications after thoracotomy. Chest 2005;127:1977-83. [Crossref] [PubMed]
- Crotty Alexander LE, Shin S, Hwang JH. Inflammatory Diseases of the Lung Induced by Conventional Cigarette Smoke: A Review. Chest 2015;148:1307-22. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc 2008;22:298-310. [Crossref] [PubMed]


