Mid- to long-term outcomes of bovine jugular vein conduit implantation in Chinese children
Introduction
Right ventricular outflow tract (RVOT) reconstruction surgery is widely applied to repair congenital heart defects. Among a variety of xenografts for implantation, the bovine jugular vein (BJV) conduit is considered a good replacement for homografts (1). It is cost-effective and flexible, with a wide range of sizes; therefore, it is often applied to reconstruct the RVOT in many complex cardiac malformations. Moreover, the BJV has been demonstrated to be associated with a better hemodynamic performance, lower rate of fibrocalcification, and lower antibody induction, compared to other xenografts and even homograft conduits (1-3). However, drawbacks of BJV conduits also have been noted, such as susceptibility to distal conduit stenosis; in addition, early valve incompetence and conduit thrombosis have been shown to be more prevalent in patients with BJV graft implantation (4,5). Therefore, conduit durability has been paid particular attention in Western countries; however, the relevant data and experiences in China are limited. Therefore, the aim of this study was to investigate the incidence of BJV conduit failure during a mid- to long-term follow-up period in Chinese children.
Methods
Patients
Fifty-three patients (33 males and 20 females) who underwent RVOT reconstruction with BJV conduits from January 2002 to December 2013 at the Department of Cardiovascular Surgery, Children’s Hospital of Fudan University, Shanghai, China, were recruited for this study by telephone. All patient data and prognosis information were reviewed retrospectively. All legal guardians of the patients signed consent forms prior to enrollment, and the study protocol was approved by the institutional review board of our institution (No. 2007-14).
Surgical procedures
First, cardiac malformation was repaired with heart arrest during a standard cardiopulmonary bypass. The minimal conduit size was calculated according to the patient’s weight using standard tables. The BJV conduit (BalMedic Company, Beijing, China) was tailored into a slanted shape to increase the size of the anastomosis to the pulmonary artery at its distal end. The RVOT was reconstructed with the conduit implanted with end-to-end anastomosis to the pulmonary artery. The conduit valve was implanted as close as possible to the pulmonary artery bifurcation to prevent potential regurgitation due to conduit twisting caused by blood flow filling. The proximal end of the BJV graft was cut into a redundant anterior flap to construct the hood of the outflow tract and anastomosed with the right ventricle incision. The autologous pericardial patch was added as a hood for the conduits smaller than 14 mm to prevent proximal anastomotic stenosis. Cefazolin (50 mg/kg) was given intravenously before sternal incision and continued every 12 h for 3 days. Heparin (5–15 units/kg/h) was given continuously and intravenously at 4–5 h postoperatively. Then, oral aspirin was administered to replace heparin when the patients were transferred to the ward; aspirin therapy was continued for 2 years after the operation.
Demographic and perioperative information
Patient information, including age, gender, previous surgeries, and heart defect diagnosis, was collected at the time of admission. Perioperative information, such as cross clamp time, cardiopulmonary bypass time, ventilation duration, intensive care unit duration, and bleeding volume, was recorded. Active bleeding was diagnosed if the bleeding volume was more than 5 mL/h/kg within 3 h. Post-operation complications, including delayed sternal closure, low cardiac output syndrome, or complete atrioventricular block, were also diagnosed.
Conduit failure
Conduit stenosis was defined as a peak instantaneous conduit gradient greater than 60 mmHg by echocardiography (6). Conduit failure was defined as conduit stenosis, greater than grade 3+ regurgitation requiring surgical conduit replacement, or transcatheter conduit dilatation (7).
Endocarditis
Endocarditis was defined by new conduit vegetation visualized on echocardiography or by two positive blood culture results (8). Treatment mainly included antibiotic therapy for at least 6–8 weeks until negative blood culture results. If conduit stenosis due to endocarditis was significant, conduit replacement was recommended.
Statistical analysis
Statistical analyses were performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). Continuous variables were presented as the mean ± standard deviation (SD) for normally distributed data or as the median and interquartile range otherwise. For subgroup analyses, the Student’s t-test was used to compare parametric variables, and the Mann-Whitney U test was used to compare nonparametric variables. The BJV conduit failure rate was analyzed using Kaplan-Meier analysis. Potential risk factors for BJV conduit failure were analyzed, and variables with P values less than 0.1 were assessed using multivariate logistic regression to evaluate risk factors for BJV conduit failure. A probability (P) value of <0.05 was considered to be significant.
Results
Patient characteristics
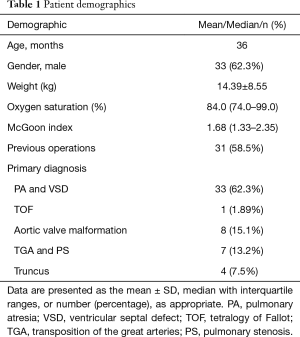
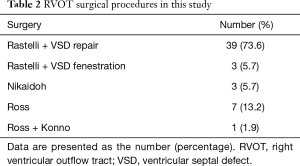
Overall, 53 patients (33 males and 20 females) underwent 55 BJV conduit implantations. The median age of all patients was 36 months old (range, 1–142 months old), and the average weight was 14.4±8.6 kg (3.2–35 kg). Patient demographics and primary diagnoses are summarized in Table 1. More than half of the patients (58.5%) had previous palliative surgery, including 29 modified Blalock-Taussig shunts and 2 right ventricle-to-pulmonary shunts. RVOT surgical procedures were listed in Table 2.
Full table
Full table
Peri-operational outcomes
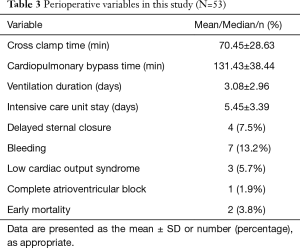
Two patients died at the early stage after conduit implantation. The causes of death were severe valve conduit regurgitation due to a high pulmonary resistance and uncontrolled endocarditis, respectively. Of all the patients, four patients had delayed sternal closure, and seven patients had severe bleeding, among which two patients required additional emergency surgery. In addition, three patients presented with low cardiac output symptoms, among which one patient needed peritoneal dialysis, and one patient was implanted with a permanent pacemaker due to an unrecovered complete atrioventricular block (Table 3).
Full table
Post-operational outcome
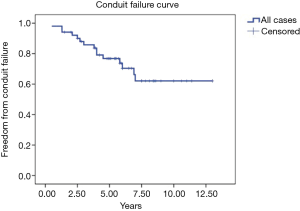
Fifty-one patients were followed up completely. The median follow-up period for these patients was 6.3 years (interquartile range, 4.9–8.6 years). Fifteen of these patients (29.4%) were diagnosed with conduit failure due to conduit stenosis (n=10), stenosis and regurgitation (n=3), and regurgitation alone (n=2). Kaplan–Meier analysis showed that the proportion of patients who were free from BJV conduit failure was 98.0%, 85.8%, 76.8%, and 62.1% at 1, 3, 5, and 7 years, respectively (Figure 1). Two patients with BJV conduit failure underwent conduit replacement, and another four patients were partially relieved through balloon dilatation by catheter. The other nine patients were scheduled for surgery or catheter intervention.
Risk factors for BJV conduit failure
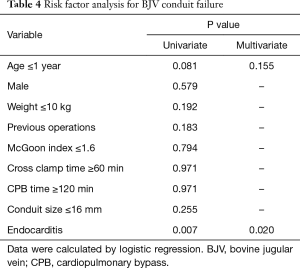
Variables that potentially correlated with BJV conduit failure were analyzed by logistic regression. Univariate analysis revealed that an age less than one year (P=0.081) and endocarditis (P=0.007) were possible risk factors for BJV conduit failure. After multivariate analysis, only endocarditis was a significant risk factor for BJV conduit failure (OR: 6.735; 95% CI: 1.348–33.647) (Table 4).
Full table
Endocarditis
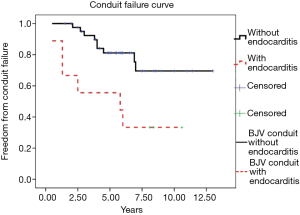
After conduit implantation, 9 of 51 patients were diagnosed with endocarditis at the median follow-up period of 25.3 months (10 days to 69 months). Seven patients were diagnosed by positive blood culture results(6 Staphylococcus aureus and 1 Streptococcus viridans), whereas the other two were confirmed with conduit vegetation by echocardiography. After several weeks of antibiotic treatment, all patient blood culture samples tested negative. Conduit failure was also confirmed in six of the nine patients. One patient had undergone conduit replacement. Kaplan-Meier analysis demonstrated that the proportion of patients with endocarditis who were free from BJV conduit failure was significantly lower than that of patients without endocarditis (P=0.007) (Figure 2).
Discussion
This study evaluated the mid- to long-term outcomes after BJV conduit implantation and demonstrated that endocarditis was the most significant risk factor for BJV conduit failure. To the best of our knowledge, this is the largest study to report a single-center series of patients for up to 13 years in China.
The overall prognosis of BJV conduit implantations has not reached a consensus, and most studies have only reported medium-term follow-up data. A recent study by Sandica et al. has shown that the BJV was a better homograft in terms of its durability after their follow-up study of 27 years (9). On the contrary, Ugaki et al. have demonstrated an increased incidence of post-operation endocarditis in BJV-implanted patients than in patients with homografts (10). Therefore, understanding the long-term prognosis of BJV conduit implantations will greatly help surgeons to decide the best graft to use in clinical settings.
Our study demonstrated a relatively high rate of BJV conduit failure (29.4%) despite the fact that large-size conduits were used. Niemantsverdriet et al. (11) have shown that the rate of conduit failure is up to 50% at 5 years after BJV conduit implantation, according to their multinational analysis in Europe and the United States from 1987 to 2003. It seems that conduit failure has decreased according to recent reports, but it remains at 20% at 5 years and 40% at 10 years after surgery (10). Conduit failure can lead to two or more surgeries in many patients (12). Therefore, the high rates of conduit failure and a second surgery in the current BJV application were inevitable during the long-term follow-up period.
Our study also demonstrated that endocarditis occurred in 17.6% of BJV conduit implantations, which was slightly higher than reported previously (7–11%) (10,13,14). In addition, it has been reported that BJV conduits are more likely to result in endocarditis compared to homografts (<1%) (11). In our study, the earliest that endocarditis happened was at 10 days after surgery, whereas most cases of endocarditis occurred at 6 months after conduit implantation. The mechanism still remains unclear. Ugakiet al. (10) have suggested that endocarditis is due to randomly occurring bacteremia from routine daily activities. In addition, Mery et al. (14) have indicated that BJV conduits cause endocarditis due to an immunologic/inflammatory reaction related to the jugular vein. The most common organism detected by blood culture was methicillin-sensitive S. aureus, which could be well controlled by vancomycin if given on time. Our study also confirmed that endocarditis accelerated BJV conduit deterioration. Vegetation and conduit stenosis were commonly observed in failed BJV conduits. The worst situation occurred in BJV conduit obstruction, which usually required emergency surgery or extracorporeal membrane oxygenation support (15), and was considered one of the main reasons for late mortality (16). In terms of the other possible risk factors for BJV conduit failure, our study did not find that either a younger age (≤1 year) (17) or a small-size conduit (≤14 mm) (18) was a significant risk factor, possibly because we used a different study population and a larger BJV conduit size than other studies. The mechanism of BJV conduit-related endocarditis and risk factors require more investigations.
Other potential reasons for BJV conduit failure include thrombosis, calcification, and immunologic/inflammatory reactions. More evidence supports that the immunologic/inflammatory reaction plays a role in the process of BJV conduit failure. Wojtalik et al. (19) have demonstrated a B-cell increase of up to 150% of the normal value with T-lymphocyte activation and the presence of CD69+ and CD71+ cells at 3 to 6 months postoperatively. In addition, Schoenhoff et al. (2) have detected a chronic inflammatory lesion through the histopathological examination of a failed BJV conduit as well as a large number of monocytes and macrophagocytes as reported by Park et al. (20). Moreover, Rüffer et al. (6) have found that patients with a high white blood cell count at the 8th postoperative day have a high risk for BJV conduit failure. Therefore, the current opinion suggests that the degeneration of BJV conduits is inevitable as they are regarded as a prosthetic valve conduit (20).
The present study is limited by its retrospective and nonrandomized design. The number of patients is relatively small and the range of age and weight at BJV implantation is relatively wide. It confirmed that frequent endocarditis occurred in the BJV conduits. Potential residual confounding was also possible in this study.
In conclusion, the durability of BJV conduits is suboptimal after mid- to long-term follow-up, and conduit stenosis is often detected. In addition, endocarditis is a significant risk factor that accelerates BJV conduit deterioration. Future large-scale and prospective studies with a longer follow-up period are warranted.
Acknowledgements
Funding: This work was supported by National Key Clinical Specialty Construction Programs of China [2014–2016]; and Medical Guide Project of Shanghai Municipal Science and Technology Commission, No. 134119a4100.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All legal guardians of the patients signed consent forms prior to enrollment, and the study protocol was approved by the institutional review board of our institution (No. 2007-14).
References
- Fiore AC, Brown JW, Turrentine MW, et al. A bovine jugular vein conduit: a ten-year bi-institutional experience. Ann Thorac Surg 2011;92:183-90; discussion 190-2. [Crossref] [PubMed]
- Schoenhoff FS, Loup O, Gahl B, et al. The Contegra bovine jugular vein graft versus the Shelhigh pulmonic porcine graft for reconstruction of the right ventricular outflow tract: a comparative study. J Thorac Cardiovasc Surg 2011;141:654-61. [Crossref] [PubMed]
- Brown JW, Ruzmetov M, Rodefeld MD, et al. Valved bovine jugular vein conduits for right ventricular outflow tract reconstruction in children: an attractive alternative to pulmonary homograft. Ann Thorac Surg 2006;82:909-16. [Crossref] [PubMed]
- Prior N, Alphonso N, Arnold P, et al. Bovine jugular vein valved conduit: up to 10 years follow-up. J Thorac Cardiovasc Surg 2011;141:983-7. [Crossref] [PubMed]
- Vitanova K, Cleuziou J, Hörer J, et al. Which type of conduit to choose for right ventricular outflow tract reconstruction in patients below 1 year of age? Eur J Cardiothorac Surg 2014;46:961-6; discussion 966. [Crossref] [PubMed]
- Rüffer A, Wittmann J, Potapov S, et al. Mid-term experience with the Hancock porcine-valved Dacron conduit for right ventricular outflow tract reconstruction. Eur J Cardiothorac Surg 2012;42:988-95. [Crossref] [PubMed]
- Kalfa D, Feier H, Loundou A, et al. Cryopreserved homograft in the Ross procedure: outcomes and prognostic factors. J Heart Valve Dis 2011;20:571-81. [PubMed]
- Meyns B, Van Garsse L, Boshoff D, et al. The Contegra conduit in the right ventricular outflow tract induces supravalvular stenosis. J Thorac Cardiovasc Surg 2004;128:834-40. [Crossref] [PubMed]
- Sandica E, Boethig D, Blanz U, et al. Bovine Jugular Veins versus Homografts in the Pulmonary Position: An Analysis across Two Centers and 711 Patients-Conventional Comparisons and Time Status Graphs as a New Approach. Thorac Cardiovasc Surg 2016;64:25-35. [PubMed]
- Ugaki S, Rutledge J, Al Aklabi M, et al. An increased incidence of conduit endocarditis in patients receiving bovine jugular vein grafts compared to cryopreserved homograft for right ventricular outflow reconstruction. Ann Thorac Surg 2015;99:140-6. [Crossref] [PubMed]
- Niemantsverdriet MB, Ottenkamp J, Gauvreau K, et al. Determinants of right ventricular outflow tract conduit longevity: a multinational analysis. Congenit Heart Dis 2008;3:176-84. [Crossref] [PubMed]
- Ong K, Boone R, Gao M, et al. Right ventricle to pulmonary artery conduit reoperations in patients with tetralogy of fallot or pulmonary atresia associated with ventricular septal defect. Am J Cardiol 2013;111:1638-43. [Crossref] [PubMed]
- Malekzadeh-Milani S, Ladouceur M, Iserin L, et al. Incidence and outcomes of right-sided endocarditis in patients with congenital heart disease after surgical or transcatheter pulmonary valve implantation. J Thorac Cardiovasc Surg 2014;148:2253-9. [Crossref] [PubMed]
- Mery CM, Guzmán-Pruneda FA, De León LE, et al. Risk factors for development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. J Thorac Cardiovasc Surg 2016;151:432-9, 441.e1-2.
- Vohra HA, Jones C, Viola N, et al. Use of extra corporeal membrane oxygenation in the management of sepsis secondary to an infected right ventricle-to-pulmonary artery Contegra conduit in an adult patient. Interact Cardiovasc Thorac Surg 2009;8:272-4. [Crossref] [PubMed]
- Yong MS, Yim D, d'Udekem Y, et al. Medium-term outcomes of bovine jugular vein graft and homograft conduits in children. ANZ J Surg 2015;85:381-5. [Crossref] [PubMed]
- Cleuziou J, Vitanova K, Kasnar-Samprec J, et al. Durability of down-sized homografts for the reconstruction of the right ventricular outflow tract. Eur J Cardiothorac Surg 2016;49:1421-5. [Crossref] [PubMed]
- Palma G, Mannacio VA, Mastrogiovanni G, et al. Bovine valved venous xenograft in pulmonary position: medium term evaluation of risk factors for dysfunction and failure after 156 implants. J Cardiovasc Surg (Torino) 2011;52:285-91. [PubMed]
- Wojtalik M, Mrowczynski W, Zeromski J, et al. Does contegra xenograft implantation evoke cellular immunity in children? Interact Cardiovasc Thorac Surg 2003;2:273-8. [Crossref] [PubMed]
- Park CS, Park SS, Choi SY, et al. Anti alpha-gal immune response following porcine bioprosthesis implantation in children. J Heart Valve Dis 2010;19:124-30. [PubMed]


