Neoadjuvant treatment of locally advanced esophageal and junctional cancer: the evidence-base, current key questions and clinical trials
Introduction
Despite major advances in the multimodal approach to locally advanced esophageal and junctional cancer, the 5-year survival of patients treated with curative intent remains poor, at between 30% to 47% in recent series (1-4). Neoadjuvant therapy, either combination chemotherapy and radiation therapy (multimodal), preoperative chemotherapy, or pre-and post-operative chemotherapy, has become more standard based on randomized clinical trials (RCTs) compared with surgery alone. The goal of neoadjuvant therapy is to increase resection rates, including complete (R0) rates, and to reduce local and systemic recurrence with consequent improved disease-specific and overall survival. Based on R0 rates, histopathologic responses, including pathologic complete responses (pCR), and nodal downstaging, the primary impact of multimodal regimens is on the tumor and regional nodes, wheras chemotherapy alone has both local effects, albeit more modest than chemoradiation, as well as systemic effect on minimal residual disease (5-7). No neoadjuvant approach is without risk, including toxicities specific to the drugs or radiation therapy, and potential increased risk of postoperative morbidity and mortality. Moreover, some patients will be completely resistant to therapy and may progress during the approximate three month period from initiation of neoadjuvant therapy to planned surgery. Notwithstanding, at this time neoadjuvant therapy has supplanted surgery alone as primary therapy with curative intent for locally advanced esophageal and junctional tumors.
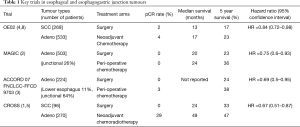
The key published RCTs that inform current practice are shown in Table 1. For multimodal therapy, prior to the CROSS (Chemoradiotherapy for Esophageal Cancer followed by Surgery Study) trial, RCTs tended to be small and underpowered, inclusive of both adenocarcinoma (EAC) and squamous cell (SCC) subtypes, with large variation in dose and fractionation, with just one completed trial, published in 1996, of 113 patients with adenocarcinoma, showing a highly significant improvement in 3-year survival (32% vs. 6%; P=0.01) compared with surgery alone (9). The CROSS Trial defined a new benchmark across esophageal oncology, where in a study of 366 patients, 75% with adenocarcinoma, multimodal therapy (paclitaxel, carboplatin and 41.4 Gy/23 fractions) resulted in 92% complete resection rate (R0), a complete pathologic response rate (pCR) of 29%, and a median overall survival of 49 months compared with 24 months (95% CI: 0.49–0.87, P=0.003) compared with surgery alone. The 5-year overall survival of 47% far exceeds that previously reported in RCTs, and there was no evidence of increased postoperative complications from this regimen. Moreover, side-effects of the protocol were few, 13 (8%) had grade 3 or worse haematological toxicity, and 18 (11%) had grade 3 or worse non-haematological toxicity. Longer follow up showed reduced locoregional recurrences in the multimodal arm, and to a lesser extent reduced systemic recurrences (1). The other strongly positive modern RCT was the CALBG 9781, where (n=56 of planned 540) patients treated with 5-FU/Cisplatin and 50.4Gy RT had a 5 year overall survival of 39% compared with 16% in surgery only (P<0.008), with a pCR of 40% (10). Although clearly underpowered, the impact was significant in North American practice where this protocol is commonly utilized. For neo-adjuvant or perioperative chemotherapy, there are four key trials compared with surgery alone. The RTOG 8911/ Intergroup 0113 RCT randomised 440 patients, 54% with adenocarcinoma, to pre- and postoperative 5-Fluorouracil and cisplatin, or surgery alone. No improvement in survival was evident (11). A similarly powered study of 802 patients conducted by the Medical Research Council in the UK, the OEO2 Trial, where 66% of patients had adenocarcinoma, and patients were randomised to two cycles of pre-operative Cisplatin and 5-FU, or surgery alone. The treatment arm had a 23% 5-year overall survival compared with 17% in the surgery alone group (HR =0.84; 95% CI, 0.72–0.98, P=0.03) (4,8). The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial of 503 patients, although powered for gastric adenocarcinoma, included 11% with junctional and 14% with lower esophageal adenocarcinoma, and compared 3 cycles of epirubicin, Cisplatin and 5-FU (ECF) before and after surgery with surgery alone (2). Grade 3-4 hematologic toxicities were evident in 24% of patients, and just 55% started and 42% completed postoperative chemotherapy. Pathologic down-staging in tumor and nodal sites were evident, and the 5-year survival rate was 36% for combined modality therapy compared with 23% for patients with surgery alone (P=0.009). Of note, the therapy efficacy was independent of the tumor site. The French ACCORD-07 provided similar results, recruiting 224 of a planned 250 patients, with 64% having junctional adenocarcinoma, and 11% with lower esophageal adenocarcinoma. Two pre- and four postoperative cycles of Cisplatin and 5-FU were given, and the 5 year survival was 38% for combination therapy compared with 24% for surgery alone (P=0.02) (3). Accordingly, the MAGIC and ACCORD trials together provide a significant level of evidence for perioperative chemotherapy in adenocarcinoma of the lower esophagus and junction compared with surgery alone. Of additional interest, in Japan, where adjuvant chemotherapy was standard of care for stage II or II esophageal squamous cell carcinoma based largely on the results of the RCT JCOG 9204 (12), a trial of 330 patients comparing 2 cycles of neoadjuvant cisplatin and 5-FU versus post-operative CF in this population was terminated early as the 5 year overall survival in the neoadjuvant group were superior to that of the adjuvant group [55% vs. 43% HR: 0.73 (0.54–0.99), P=0.04].
Full table
The recent publication of the Phase II component of the FLOT4 study, in patients with gastric or junctional adenocarcinoma, is also of major current interest. In this study of 300 patients from 28 German centers, which compared ECF/ECX (3 cycles pre and post operatively) with docetaxel, oxaliplatin, 5-FU and leucovorin (FLOT), 4 cycles pre and 4 postoperatively, junctional cancers, including adenocarcinoma of the esophago-gasric junction (AEG) type I, II and III, represented 57% of the ECF/X cohort, and 48% of the FLOT cohort (13). The primary end point was pCR, this was 16% for the FLOT regimen compared with 6% for ECF/ECX. This highest reported pCR with chemotherapy alone, and an overall major pathological response rate of 37% with FLOT highlights that this regimen may result in biological effects at the primary site that approach what is observed with neoadjuvant chemoradiation, and forms the basis for the ESOPEC trial (vide infra). Also of interest is the results from the OE05 trial, published in abstract format (14). This trial randomised 897 patients with lower esophageal or junctional (AEG I and II) tumours to 2 cycles of cisplatin/5-FU (CF) and surgery versus 4 cycles epirubicin/cisplatin/capecitabine (ECX) followed by surgery. There no differences in overall survival at 3 years between the arms [CF: 39% (35–44%) vs. ECX: 42% (37–46%)]. There were more complete responses and a longer interval to disease recurrence in the ECX arm but there were more grade 3 toxicities (47% vs. 30%, P<0.001). 89% completed more than 3 cycles of ECX compared with 96% for CF.
Key current questions and active trials
Question 1: What approach, multimodal or chemotherapy-only, is superior in the neoadjuvant or perioperative approach to locally advanced esophageal and junctional cancer?
Whereas the superiority of multimodal therapy or neoadjuvant or perioperative chemotherapy to surgery alone is firmly established from Level 1 evidence, in contrast, no conclusions can be drawn from the limited data on direct comparisons between the two. The Preoperative Chemotherapy or Radiochemotherapy in Esophagogastric Adenocarcinoma Trial (POET) sought to address this question, where 119 patients with EUS staged (uT3-4, Nx, Mo) adenocarcinoma (AEG 1and II) received either preoperative induction chemotherapy (Cisplatin, 5-FU and leucovorin) or induction chemotherapy followed by chemoradiation [Cisplatin and etoposide with 30Gy in 15 fractions of radiation therapy (RT)], and then surgery. The trial was closed prematurely due to slow accrual. Interestingly, the 3 year survival in the multimodal arm of 47.4% compared with 27.7% in the chemotherapy arm (P=0.07), the pCR rate was 15.6% in the multimodal group compared with 2% in the chemotherapy group (P=0.03), and the pathological node negative rate was also significantly decreased (64.4% vs. 37.7%; P=0.01). The most recent updated meta-analysis of RCTs for esophageal cancer, published in 2011, concluded that “a clear advantage of neoadjuvant chemoradiotherapy over neoadjuvant therapy has not been established” (15). Moreover, a recent RCT from Scandanavia (NeoRes; NCT01362127) of 181 patients, 131 with adenocarcinoma, and powered on pCR, compared 3 cycles of CF alone with this in combination with RT (40Gy). Although pCR was 28% in combination therapy, compared with 9% in chemotherapy alone (P=0.002), with corresponding nodal metastases of 35% and 62% (P=0.001), respectively, there was no difference in overall survival between groups, and in fact an increase of in-hospital mortality (6% vs. 3%) with multimodal therapy (16,17). A multicenter propensity- matched series of 608 patients also questions any superiority of one approach, with 3-year survival of 57.9% multimodal vs. 53.4% with chemortherapy alone (HR=0.89, 95% CI: 0.67–1.17; P=0.0391) (18). Only prospective RCTs powered on survival will answer this question, and in this context, new trials have been developed to compare neodjuvant chemotherapy with multimodal therapy, particularly using the CROSS regimen as the multimodal benchmark, and focused more on adenocarcinoma than squamous cell cancer (SCC), who clearly have different biology particularly with respect to response to radiation therapy. This differential biology is highlighted in the CROSS trial, where of the 42 patients with SCC treated with chemoradiotherapy, the median survival was 81.6 months (95% CI: 47–118), compared with 43.2 months (95% CI: 29.9–61.4) in 134 patients with adenocarcinoma, with complete pathologic response rates of 49% and 23%, respectively. Moreover, the separation of outcome between the multimodal regimen compared with surgery alone was highly significant [P=0.011; HR=0.45 (95% CI: 0.24–0.84)] for SCC, but less so for EAC [(P=0.049) HR =0.73 (95% CI: 0.52–0.99)], highlighting a greater rationale for trials that challenge ;multimodal therapy in adenocarcinoma (5).
Current trials are aimed at comparing current neoadjuvant chemotherapy versus chemoradiotherapy regimes in phase III studies (Neo-AEGIS. ESOPEC, TOP GEAR), as well as phase II studies aiming to explore the optimal components of new neoadjuvant/peri-operative chemotherapy or chemoradiotherapy regimes versus current practice (NeoScope, PROTECT 1402). Modern trials are enabled by most recent update of the TNM staging system (AJCC/UICC 7th edition) for cancer of the esophagus reclassified cancer arising within 5cm of the gastro-esophageal junction as esophageal, whereas heretofore AEG III, arising below the junction but involving it was viewed as gastric, and the classification of nodal disease for all esophageal and junctional cancer is now uniform and based on number of lymph nodes involved . Heretofore, AEG I and II were included in esophageal-focused trials such as CROSS, and AEG III in gastric trials.
Neo-AEGIS (NCT01726452) is a multicenter phase III open-labelled, randomised controlled trial in patients with adenocarcinoma of the esophagus and junction, including AEG III, comparing CROSS with a modified MAGIC perioperative regimen, with capecitabine and oaxilplatin accepted as alternatives to 5-FU and cisplatin, as per the REAL 2 trial (19,20). This international trial, co-ordinated by Cancer Trials Ireland, includes centers from the UK, Ireland, and Denmark, and is the first to include PET-CT as the uniform standard baseline staging modality, and uniform classification of complications as per a recent international consensus (21). Strict radiation therapy quality assurance and audit is also embedded in the protocol. The trial, activated in 2014, is powered on a 10% superiority in 3 year survival with CROSS vs. MAGIC (53% vs. 43%), with 540 evaluable patient.
ESOPEC (NCT02509286) is a prospective multicenter phase III trial from 16 German trial centers which compares the CROSS regimen with peri-operative FLOT regimen chemotherapy (4 cycles pre and post) in adenocarcinoma of the esophagus and junctional. Again, similar to neo-AEGIS, it includes all junctional tumors including AEG type III. The trial, activated in March 2016,aims to recruit 438 participants with adenocarcinoma of the lower esophagus or junction (stage Ib –IIIc) with the primary end point being overall survival at 36 months (22). In contrast to neo-AEGIS, the power calculation is based on the FLOT arm being superior, with 3-year survival of 68% based on published and unpublished experience, compared with 55% for the published CROSS trial (23).
TOPGEAR, activated in 2009, is led by the Australasian Gastrointestinal Tumors Group, and includes 61 active centers, including 28 in Europe through partnership with the EORTC (NCT01924819). This trial compares peri-operative (3 pre-op +3 post-op cycles of ECF/ECX) versus perioperative chemoradiation therapy [induction 2 cycles ECF, then RT (45Gy) and 5-FU, and 3 cycles ECF/ECX post-op] in gastric and junctional tumors (stage Ib-IIIc, includes AEG II and III, not AEG I) (24). It aims to recruit 752 patients, and is powered on a projected increase in 5-year survival rate from 40% from chemotherapy alone to 50% with combination therapy. A recent interim report from the first 120 patients randomised showed no difference in toxicity between the two arms, with grade 3 or greater toxicity observed in approximately 20% in each arm with 85-90% of patients progressing to surgery (25)
Other active Phase II trials are important on this theme. In NeoSCOPE (NCT01843829), the choice of an optimal chemotherapy regimen to pair with radiation therapy (45 Gy) is examined in a phase II trial of oxaliplatin/capecitabine (OXCAP-RT) versus carboplatin/paclitaxel (CarPac-RT) (26). It is a “pick a winner” design based on pCR,, with 38 patients with esophageal or junctional adenocarcinoma (AEG type I/II; ≥T3±≥N1) per treatment arm, the trial will be used to define the optimal regimen for a future phase III trial comparing neoadjuvant chemotherapy. Similarly, the PROTECT-1402 French trial (NCT02359968) is a phase II trial which will randomise 106 patients with stage II or III esophageal adenocarcinoma or squamous cell carcinoma to one of two neoadjuvant chemotherapy regimens with standard concurrent 41.4 Gy radiation (27). The chemo regimens are 3 cycles of FOLFOX or carboplatin/paclitaxel. The primary outcomes will be the R0 resection rate and severe post-operative morbidity. The Canadian POWERRANGER (NCT01404156) trial is a phase II trial aiming to recruit 60 participants randomised to either 3 cycles of pre-op ECF or carboplatin/paclitaxel with concurrent 45 Gy radiotherapy. The primary endpoints include compliance with the treatment regimen and assessment of pathological response (28).
In contrast to adenocarcinoma, relatively few new trials for SCC are active. A phase III RCT comparing neoadjuvant chemotherapy with cisplatin and paclitaxel versus surgery alone aims to recruit 528 patients in China with IIa-IIIb esophageal squamous cell carcinoma (NCT02395705) (29). A three arm neoadjuvant trial entitled the NExT study (JCOG1109, NCT00525915), began recruitment in Japan in 2012 and aims to recruit 501 patients with stage Ib-IIIc (excluding T4) squamous cell cancer (30). It compares neoadjuvant docetaxel/cisplatin/5-FU versus cisplatin/5-FU/concurrent RT versus cisplatin/5-FU. This trial will assess the efficacy of chemoradiation regimes in Eastern populations undergoing more extensive transthoracic lymphadenectomy then has been standard in Western trials.
Question 2: trials of targeted therapies and immune based approaches in esophageal cancer
The ToGA trial is the one published trial in upper gastrointestinal cancer, in patients with HER-2-positive metastatic gastric and junctional cancers, where targeted therapy with Herceptin has impacted on outcome and is included in current treatment guidelines (31). In patients treated with curative intent, a National Cancer Institute phase III trial aims to evaluate the addition of trastuzumab to neoadjuvant chemoradiotherapy in HER-2 overexpressing esophageal adenocarcinoma in 591 patients with Stage Ib-IIIc disease (NCT01196390). The chemoradiation regime consists of carboplatin/paclitaxel with concurrent radiotherapy. A German, industry-sponsored phase II trial of the addition of adjuvant trastuzumab to peri-operative FLOT (4+4 cycles) in HER2-positive locally advanced, resectable cancers of the esophagogastric junction and stomach has completed recruitment (HerFLOT, NCT01472029). The primary endpoint is the complete pathological response rate in 53 patients which will be used to determine the feasibility of a phase III trial(32). A feasibility study (ST03, ISRCTN46020948) looking at lapatinib for HER2 positive cancer is still open. In the substantive part of this ST03 trial, patients with esophageal and junctional adenocarcinoma of stomach or junction were randomised to ECX chemotherapy or ECX in combination with the VEGF inhibitor bevacizumab, for 3 cycles before and after surgery, with a further 6 maintenance doses in the bevacizumab arm. Although no differences in survival were reported, a high anastomotic leak rate (23%) in patients undergoing esophageal surgery on the experimental arm, compared with 9% in the ECX arm, with 30 day mortalities of 11% and 5%, respectively, resulting in closure of recruitment to patients with esophageal and junctional tumors in 2013 (33)
With respect to immunotherapy, an industry sponsored phase III trial will randomise patients who have undergone neoadjuvant chemoradiotherapy and surgery to adjuvant nivolumab or placebo (Checkmate 577, NCT02743494) (34). Nivolumab is a human IGG4 anti-PD-1 monoclonal antibody which acts as a checkpoint inhibitor which prevents inactivation of anti-tumour T cells (35). Only patients with residual disease after resection will be eligible. Stage II/II cancers of the esophagus and junction, of both histological subtypes are included. In the trial design, 760 patients will be randomised, and the primary endpoint is disease-free survival at 29 months, and overall survival at 42 months.
Question 3: Does cT2N0 represent a disease stage that justifies inclusion in trials of locally advanced disease?
For CROSS and CALBG trials, and the current ESOPEC and NeoAEGIS, patients with cT2N0 disease are included (5,9,10). However it is uncertain from published trials whether patients with this disease stage derive any benefit from neoadjuvant therapy. Conversely, the French FFCD 9901 trial randomly assigned 195 patient with cT1,2, Nany or cT3 N0 tumors to pre-operative 5-FU and cisplatin and concurrent 45 Gy RT, versus surgery alone (36). The trial was stopped early as the planned enrolment would not show a significant benefit in favour of one arm over the other. NeoCRT was associated with increased peri-operative mortality (11.4% vs. 3.4%). In addition, a multicenter European collaboration including 355 patients with cT2N0 disease and propensity matching showed no benefit to multimodal therapy compared with surgery alone (18). Consequently, at this point it is reasonable to conclude that there is no compelling evidence to support neoadjuvant therapy in this cohort, notwithstanding the limitations of even modern staging with EUS and CT-PET with understaging in approximately half of patients and overstaging in about one quarter of tumors in this group (37,38). RCTs powered on predicted node negative disease in adenocarcinoma and including surgery only arms would be of interest and should be developed.
Question 4: Is there a role for a “watch and wait” policy in patients with a predicted complete clinical response following neoadjuvant therapy?
An important question is whether patients who have apparently no evidence of residual disease after neoadjuvant therapy, in particular chemoradiation, can be kept under close surveillance and only undergo surgery, so-called salvage resections, if evident localised tumor re-presents. This model is well developed in rectal cancer, for instance in a recent series of 129 patients on a “watch and wait” protocol after a complete clinical response to chemoradiation, 34% relapsed, with 36 or 41 salvaged by surgery, and of the remainder, the 3-year disease-free survival was 88 percent (39). Whether such a model translates to esophageal cancer is unknown, but if so this would have the clear advantage of sparing the patient an operation that carries a significant risk of major morbidity, and up to a 5% mortality risk, as well as enabling organ preservation and likely improved quality of life compared with that post resection. This question is currently being addressed in the Dutch pre-SANO trial (Surgery As Needed in Esophageal cancer) which may progress to a randomized trial, SANO (40), A similar study is developed in France, the Esostrate-Prodige 32 study (41). In Pre-SANO, which has almost completed its recruitment target of 215 patients, patients with an apparent complete clinical response at 6 weeks based on endoscopy, deep biopsies, CT and EUS, will be reassessed at 12 weeks, with a CT-PET in addition, prior to surgical resection. It is hoped that the predictive value of pre-SANO will be high, so that the SANO RCT can be designed, with anticipated 300 patients, randomised to a strict surveillance protocol or resection. Based on current knowledge, however, sequential CT-PET and EUS is limited by poor sensitivity and positive predictive value, and the majority of patients thought to have a complete pathological response have residual tumour at pathological analysis (42,43). Hence the outcome of the pre-SANO study and the Esostrate studies under strictly controlled conditions are awaited with great interest.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shapiro J, van Lanschot JJB, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon J-P, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Samson P, Robinson C, Bradley J, et al. Neoadjuvant Chemotherapy versus Chemoradiation Prior to Esophagectomy: Impact on Rate of Complete Pathologic Response and Survival in Esophageal Cancer Patients. J Thorac Oncol 2016;11:2227-37. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A Comparison of Multimodal Therapy and Surgery for Esophageal Adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007;25:3719-25. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Schulz C, Kullmann F, Kunzmann V, et al. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma—Very good response predominantly in patients with intestinal type tumors. Int J Cancer 2015;137:678-85. [Crossref] [PubMed]
- Alderson D, Langley RE, Nankivell MG, et al. editors. Neoadjuvant chemotherapy for resectable oesophageal and junctional adenocarcinoma: Results from the UK Medical Research Council randomised OEO5 trial (ISRCTN 01852072). ASCO Annual Meeting Proceedings; 2015.
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Klevebro F, Johnsen G, Johnson E, et al. Morbidity and mortality after surgery for cancer of the oesophagus and gastro-oesophageal junction: A randomized clinical trial of neoadjuvant chemotherapy vs. neoadjuvant chemoradiation. Eur J Surg Oncol 2015;41:920-6. [Crossref] [PubMed]
- Klevebro F, von Döbeln GA, Wang N, et al. A randomised clinical trial of neoadjuvant chemotherapy vs. neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 2016;27:660-7. [Crossref] [PubMed]
- Markar SR, Noordman BJ, Mackenzie H, et al. Multimodality treatment for esophageal adenocaricnoma: multi-center propensity-score matched study. Ann Oncol 2017;28:519-27. [Crossref]
- Keegan N, Keane F, Cuffe S, et al., editors. ICORG 10-14: Neo-AEGIS: A randomized clinical trial of neoadjuvant and adjuvant chemotherapy (modified MAGIC regimen) versus neoadjuvant chemoradiation (CROSS protocol) in adenocarcinoma of the esophagus and esophagogastric junction. ASCO Annual Meeting Proceedings; 2014.
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016;16:503. [Crossref] [PubMed]
- Homann N, Pauligk C, Luley K, et al. Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5‐fluorouracil, oxaliplatin and docetaxel. Int J Cancer 2012;130:1706-13. [Crossref] [PubMed]
- Leong T, Smithers BM, Michael M, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 2015;15:532. [Crossref] [PubMed]
- Leong T, Smithers B, Michael M, et al., editors. TOPGEAR: A randomized phase II/III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer. Interim results from an international, intergroup trial of the Agitg/Trog/Ncic Ctg/Eortc. European Journal of Cancer; 2015: Elsevier Sci Ltd The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, Oxon, England.
- Mukherjee S, Hurt CN, Gwynne S, et al. NEOSCOPE: a randomised Phase II study of induction chemotherapy followed by either oxaliplatin/capecitabine or paclitaxel/carboplatin based chemoradiation as pre-operative regimen for resectable oesophageal adenocarcinoma. BMC Cancer 2015;15:48. [Crossref] [PubMed]
- Messager M, Mirabel X, Tresch E, et al. Preoperative chemoradiation with paclitaxel-carboplatin or with fluorouracil-oxaliplatin—folinic acid (FOLFOX) for resectable esophageal and junctional cancer: the PROTECT-1402, randomized phase 2 trial. BMC Cancer 2016;16:318. [Crossref] [PubMed]
- Buduhan G. PreOperative Treatment With chEmotheRapy or chemoRAdiatioN in esophaGeal or gastroEsophageal adenocaRcinoma (POWERRANGER) 2011 [updated July 2016; cited 2016 01/12/16]. Available online: https://clinicaltrials.gov/ct2/show/NCT01404156?term=esophageal+neoplasms&type=Intr&phase=2&rank=79
- Li Y. Neoadjuvant Chemotherapy Versus Surgery Alone for Esophageal Squamous Cell Carcinoma NCT02395705 2015 [1/12/16]. Available online: https://clinicaltrials.gov/ct2/show/NCT02395705?term=esophageal+neoplasms&type=Intr&phase=2&rank=46
- Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 2013;43:752-5. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- ALO-Studien-gGmbh. Explorative Phase II Study of Perioperative Treatment in Patients With Adenocarcinoma of the Gastroesophageal Junction or Stomach (HerFLOT) NCT01472029 2011. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01472029
- Cunningham D, Smyth E, Stenning S, et al. Peri-operative chemotherapy & bevacizumab for resectable gastro-oesophageal adenocarcinoma: Results from the UK Medical Research Council randomised ST03 trial (ISRCTN 46020948). EJC 2015;51:S400. [Crossref]
- Squibb B-M. Study of Adjuvant Nivolumab or Placebo in Subjects With Resected Esophageal or Gastroesophageal Junction Cancer (CheckMate 577) (NCT02743494). 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02743494.
- Jin Z, Yoon HH. The promise of PD-1 inhibitors in gastro-esophageal cancers: microsatellite instability vs. PD-L1. J Gastrointest Oncol 2016;7:771-88. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Crabtree TD, Kosinski AS, Puri V, et al. Evaluation of the reliability of clinical staging of T2 N0 esophageal cancer: a review of the Society of Thoracic Surgeons database. Ann Thorac Surg 2013;96:382-90. [Crossref] [PubMed]
- Samson P, Puri V, Robinson C, et al. Clinical T2N0 Esophageal Cancer: Identifying Pretreatment Characteristics Associated With Pathologic Upstaging and the Potential Role for Induction Therapy. Ann Thorac Surg 2016;101:2102-11. [Crossref] [PubMed]
- Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016;17:174-83. [Crossref] [PubMed]
- Noordman BJ, Shapiro J, Spaander MC, et al. Accuracy of Detecting Residual Disease After Cross Neoadjuvant Chemoradiotherapy for Esophageal Cancer (preSANO Trial): Rationale and Protocol. JMIR Res Protoc 2015;4:e79. [Crossref] [PubMed]
- Dijon CHU. Comparison of Systematic Surgery Versus Surveillance and Rescue Surgery in Operable Oesophageal Cancer With a Complete Clinical Response to Radiochemotherapy (Esostrate) NCT02551458 2015 [1/12/16]. Available online: https://clinicaltrials.gov/ct2/show/NCT02551458
- Cheedella NK, Suzuki A, Xiao L, et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann Oncol 2013;24:1262-6. [Crossref] [PubMed]
- Heneghan HM, Donohoe C, Elliot J, et al. Can CT-PET and Endoscopic Assessment Post-Neoadjuvant Chemoradiotherapy Predict Residual Disease in Esophageal Cancer? Ann Surg 2016;264:831-8. [Crossref] [PubMed]


