Four arms robotic-assisted pulmonary resection—left lower lobectomy: how to do it
Introduction
Video-assisted thoracic surgery (VATS) for anatomic pulmonary resection, including lobectomy, offers significant benefits over open thoracotomy regarding shorter hospital stay, post-operative pain and complications and better quality of life for the patient after surgery and to date is worldwide accepted as the standard approach for lung resections (1). However, VATS is technically demanding. The surgeon has restricted the ability to manoeuvre long and rigid instruments and should deal with “only” two-dimensional (2D) visualisation with the lack of the eye-hand target axis. The da Vinci robotic surgical system with the three-dimensional (3D) high-definition stereoscopic camera and the endo-wrist technology, leading to intuitive tools manoeuvrability, has helped to overcome these limitations. The 3D magnified view and precise dissection allow the surgeon to virtually perform anatomical lung resection with “his own hands” (2). However, clinical benefits and long-term results of the robotic approach, compared to VATS, are still under investigation. There are no randomised trials available and retrospective or case control analysis, reported in the literature, present controversial results (3). In the last few years numerous authors have shown that robotic-assisted pulmonary lobectomy is a safe and efficient procedure for the treatment of early stage lung cancer and more recently, experienced surgeons have proven the feasibility of more complicated operations such as pneumonectomy and bronchial/vascular sleeve resection performed robotically (4,5). Several techniques have been described to carry out a robotic lobectomy. Park and colleagues in 2006 reported the successful initial results of the robotic-assisted VATS dissection approach in 34 patients. More recently Cerfolio and colleagues published the largest series on robotic lung lobectomy resections indicating the safety and effectiveness of the completely portal robotic lobectomy technique (CPRL) (6). Since the beginning of our experience with robotic lung resection, we adopted the 4-arms robotic-assisted approach described by Veronesi and Melfi in 2010 (7).
In this study, we present our technique to perform a robotic left lower lobectomy. This is presented in easy-to-follow sequential steps. We also provide an instrument preference card and high-quality video.
Clinical vignette
A 57-year-old gentleman, non-smoker, was admitted to our division with a diagnosis of a 2-cm solid lesion located in the lower lobe of the left lung. The complete patient-specific stage included total body computer tomography, PET-CT-scan and a CT guided fine needle aspiration biopsy that confirmed the malignant nature of the tumour. The final clinical stage was cT1N0M0 adenocarcinoma. The patient was scheduled for a robotic left lower lobectomy. The operation was performed using the Da Vinci Si surgical system.
Preference cards
- A 30° stereoscopic 3D high-definition camera;
- Operative Instruments: One Fenestrated Bipolar Forceps, 2 Cardiere Forceps and a permanent Cautery Hook (EndoWrist Monopolar Cautery);
- Endo GIA™ 30 mm Curved Tip Articulating Vascular/ Medium Reload with Tri-Staple™ Technology (Covidien) for named vessels;
- Alexis™ (Applied Medical) soft tissue retractor;
- Silicone vessel loops.
Surgical technique
Patient positioning
All procedures are performed under general anaesthesia with double lumen intubation to achieve one lung ventilation. The patient is placed in right lateral decubitus with the chest orientated parallel to the floor. The hips should be put at the level of the table break and flexed to obtain maximum separation of the intercostal spaces. The robot is then positioned at the head of the patient.
Port placement
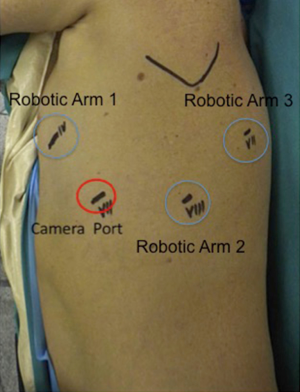
Four-arms robotic left lower lobectomy with Si da Vinci Robot is performed in the following manner. First, we c a 3-cm utility incision at the fifth intercostal space in front of the anterior border of the latissimus dorsi. In the setup process, we prefer to use a 10 mm 30° VATS camera to explore the pleural cavity and perform all the other ports under view guidance. We protect the utility incision with a soft tissue retractor (Alexis®). The camera port (12 mm port) is placed in the seventh intercostal space at the mid-axillary line usually more posteriorly than for the right side, so to be sure that the heart is out of the camera view. The next port is placed in the eighth intercostal space posteriorly; incision should be not less than 1 cm as to facilitate the introduction of the stapler trough this port. The final, fourth 8 mm incision, is made in the auscultatory triangle. Once all four trocars are in place, we start docking the Robot. Port placement and robot set up takes from 5 to 8 minutes usually. After introducing the 30-degree stereoscopic camera, we begin putting the three operative robotics arms under direct view. A Cardiere forceps is inserted through the fourth posterior trocar (Arm 3); it allows lung retraction and a better exposure of the operative field. The fenestrated bipolar forceps is placed at the level of the utility incision (Surgeon left hand: arm 1) and the permanent cautery hook in the other operative port (Surgeon right hand; arm 2) (Figure 1).
Robotic left lower lobectomy
Step 1: pulmonary ligament
After inspection of the pleural surface, to confirm the absence of metastasis, we first proceed with dissection of the pulmonary ligament. The lung is retracted from the posterior port (arm 3) with the Cardiere forceps and pulled towards the apex. This manoeuvre facilitates the exposure of the inferior pulmonary ligament. If the diaphragm dome hides the ligament, the bed assistant should introduce a sponge stick from the utility incision to push down the diaphragm so to create a larger operative room and improve visualisation of the target area. The ligament is incised with the hook diathermy all the way up to the inferior edge of the lower lobe vein. The diathermy should be applied at the junction between the lung and pleural reflection avoiding dissection of the parenchyma that will result in annoying bleeding throughout the procedure.
Step 2: left lower vein
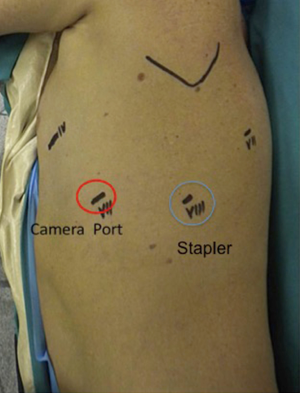
Once the inferior pulmonary vein is visualised, we always proceed to dissect the hilum anteriorly to expose and identify the upper lobe vein and confirm the presence of a normal venous anatomy. The bifurcation of the inferior and superior pulmonary veins should be dissected out. The inferior vein is then isolated and encircled with a vessel loop and then divided with at 30-mm vascular stapler introduced from the utility incision as shown in Figures 2,3. We usually prefer to complete hilar lobe isolation before proceeding with the vascular division.

Step 3: fissure division and pulmonary artery (PA) isolation
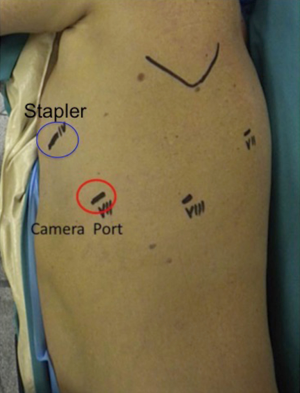
Fissure is dissected to identify the PA. Once PA is visualised, it is useful to divide the anterior portion of the oblique fissure. The interlobar lymph nodes should be dissected and removed (see Figure 3). This manoeuvre permits a proper identification of the anterior edge of the basilar trunk. Blunt dissection of the subadventitial plane is then completed to expose the PA branch for the upper segment of the lower lobe (segment S6) (see Figure 3). The PA is finally isolated and divided with a 45-mm vascular stapler. The Stapler is usually introduced from the posterior port as in Figure 4. Once the artery is divided, it is easy to complete the posterior part of the oblique fissure, in a front to back direction, with a stapler inserted through the utility incision. If the fissure is not completed, this will need to be divided to expose the ongoing interlobar artery to the lower lobe. Bipolar cautery may be used to dissect the fused fissure with only minimal post-operative air leaks.
Step 4: left lower lobe bronchus
The lobe is pulled anteriorly toward the apex by the robotic arm 3. Posterior mediastinal pleura is dissected, and all hilar lymph nodes are removed. The bronchus is then divided with a 60-mm stapler that should be passed through the utility incision but can be inserted through the arm two port if it offers a better angle. The lobe is finally pulled out of the chest through the utility incision with a specimen retrieval bag.
Step 5: lymph node dissection
- On the left side, Stations 5 and 6 are removed by pulling the upper lobe inferiorly and posteriorly with the cadiere forceps (arm 3) so to achieve a good exposure of the mediastinum and lung hilum. With a second cadiere forceps (arm 1) the mediastinal pleura should pull up and open with the cautery hook (arm 2).
- Stations 9 and 8 are usually removed during the release of the pulmonary ligament and dissection of the inferior vein.
- The subcarinal area (Station 7) requires the remaining lobe to be retracted anteriorly and superiorly. Posterior pleura should be open from the lower lobe vein stamp at the way up, along with the course of the vagus nerve, to expose the main PA. This manoeuvre facilitates identification of the target area and a safer visualisation of the oesophagus.
Tips/discussion points
- The use of the fourth arm enable stable lung retraction, and hence a better exposure of the operative field leaving two operative arms free to perform vascular e bronchial dissection. Moreover, one arm (generally the one controlled by the left hand) should be free to deal with bleeding if occurs.
- A sponge (we prefer a rolled-up sponge) should always be present on the operative field when working on vascular structures. In the case of vascular injury, the first step is to tamponade the structure with the sponge. Minor injuries may be successfully controlled by this “packing”.
- The utility incision (3 to 4 cm) at the fifth intercostal space can be converted to lateral thoracotomy very rapidly in case of uncontrolled or massive bleeding.
- Care must be taken when sliding the stapler around the PA and/or vein. It is helpful to encircle vessels with a vessel loop before passing the stapler. We prefer to use curved tip articulated vascular reload (Tri-StapleTM Technology Covidien). The curved tip facilitates to safely find the right angle to slide the stapler around the vessels.
- With the recent introduction of the Xi surgical system, new exciting tools are now available. The long tip up fenestrated grasper is particularly suggested to safely isolate and surround vascular structures and create a wide path to pass the stapler easily.
- A bed-assistant with previous experience on video-assisted thoracoscopic major lung resections is recommended particularly in the initial step of a surgeon’s learning curve. However, growing with the experience complicated procedures and vascular injuries should be managed, safely, with the “robot”.
Conclusions
In the last few years, robotic pulmonary lobectomy has been progressively adopted by a larger number of general thoracic surgeons for lung resection. The four arms robotic approach for lung lobectomy is feasible, reproducible and appears to be well suited to perform major lung resections safely. Robotic lobectomy is advantageous when compared to open lobectomy and seems to offer many of the same benefits that VATS with some additional advantages regarding dexterity, optics and surgeon ergonomics. The capital cost of the robotic surgical system is currently a significant issue. However, the economic impact of robotic devices is not less important compared to other technological innovations that are recently introduced in general thoracic practice. Moreover, robotic technology is evolving quickly. The recent introduction of new devices as the robotic stapler, suction/irrigator robotic arm and 5 mm lung forceps, have significantly improved surgeons comfort to perform more precise and safe anatomical dissection. The physicians should critically evaluate costs and benefits of any new technological devices to determine the appropriate utilisations because, as stated from Leonardo da Vinci who gave his name to the surgical robot: “I know that many will define this work as unnecessary, but apparently good guys wish to know”.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Cerfolio RJ. Pulmonary Resection in the 21st Century: The Role of Robotics. Tex Heart Inst J 2012;39:848-9. [PubMed]
- Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: state of the art and perspective. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016;101:28-34. [Crossref] [PubMed]
- Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 2016;8:S223-6. [PubMed]
- Qiu Tong, Zhao Yandong, Xuan Yunpeng, et al. Robotic-assisted double-sleeve lobectomy. J Thorac Dis 2017;9:E21-5. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Robotic-assisted pulmonary resection - Right upper lobectomy. Ann Cardiothorac Surg 2012;1:77-85. [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Pardolesi A, Bertolaccini L, Brandolini J, et al. Four arms robotic left lower lobectomy. Asvide 2017;4:258. Available online: http://www.asvide.com/articles/1567