Optical coherence tomography- vs. intravascular ultrasound-guided percutaneous coronary intervention
What was known before ILUMIEN 3
Coronary angiography is routinely used to guide percutaneous coronary interventions (PCI) despite obvious limitations of this lumen based approach. Intravascular imaging including intravascular ultrasound (IVUS) and optical coherence tomography (OCT) represent two techniques that provide essential information on pre-procedural lesion characteristics (i.e., lesion severity, landing zone, and plaque composition) and the result after stent implantation (i.e., stent expansion and eccentricity, strut apposition, lesion coverage, tissue protrusion, and dissections). A total of 11 randomized controlled trials investigated the effect of IVUS-guided PCI with mixed results (1-11). Of note, studies including patients with an increased complexity [i.e., chronic total occlusion (CTO) or lesion length >28 mm] demonstrated a consistent benefit of IVUS-guided PCI as compared with angiography, mainly driven by a reduction of repeat revascularization for restenosis (MACE at 1 year: CTO-IVUS, 2.6% vs. 7.1%, P=0.035; IVUS-XPL, 2.9% vs. 5.8%, P=0.007) (1,2). In addition, IVUS was instrumental in guiding left main stem PCI in the recent EXCEL and NOBLE left main trials in more than 70% of patients, although these studies were not designed to investigate effects of intracoronary-guided imaging (12,13).
OCT has a high spatial resolutions of 10–20 µm, which is approximately 10 times greater as compared with IVUS. Due to a lower tissue penetration, OCT is limited in determining the plaque burden, vessel size based on the detection of the external elastic membrane (EEM) at the minimal lumen diameter, which is one of the parameters used for IVUS-guided stent sizing (14). The majority of previous IVUS studies reportedly applied the multicenter ultrasound stenting in coronary (MUSIC) criteria (Table 1) (15) with the key criteria of an in-stent minimal lumen area ≥80% of the average reference lumen area or ≥90% of the lumen area of the reference segment with the lower lumen size along with symmetric stent expansion. Notwithstanding the increasing use of OCT for PCI-guidance, standard criteria for this light-based technology have not been established.
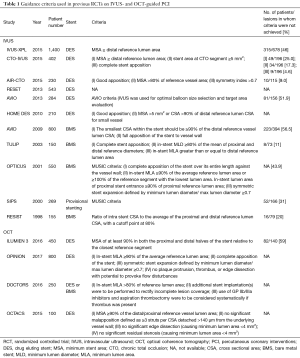
Full table
To date, the use of OCT for guiding PCI has mainly been evaluated in smaller studies, using surrogate marker endpoints (16,17). In the recent DOCTORS trial, the use of OCT-guided PCI was associated with a small but significant difference in post-procedural FFR (0.94±0.04 vs. 0.92±0.05, P=0.005) (18). In the OCTACS study, OCT-guidance resulted in a lower proportion of uncovered struts at 6 months (4.3% vs. 9.0%, P<0.01) (19).
Based on the well-established and currently still widespread clinical use of IVUS-guided PCI and the available aforementioned evidence on its effectiveness, one of the key questions is whether OCT-guided PCI using a specific protocol is comparable to IVUS-guided PCI in terms of lesion expansion. ILUMIEN 3 (20) was designed to fill this gap of clinically relevant evidence.
What the study found
ILUMIEN 3 represents the first multicenter, randomized controlled trial aiming to compare the effects of OCT-guided, IVUS-guided, and angiography guided-PCI against each other (20). Relatively simple lesions [i.e., short lesion length (median length 15.5 mm), exclusion of left main, CTO and planned 2-stent bifurcation lesion, and less than 1/3 acute coronary syndrome patients] were included to this study. The primary endpoint was minimal stent area (MSA), a measure that is closely related to the risk of future stent failures. The study found that the final MSA following OCT-guided PCI [5.79 mm2 (IQR, 4.54–7.34 mm2)] was non-inferior to that of IVUS-guided PCI [5.89 mm2 (IQR, 4.67–7.80 mm2), P for non-inferiority =0.001] but not superior to that of angiography-guided PCI [5.49 mm2 (IQR, 4.39–6.59 mm2), P=0.12]. Conversely, minimum and mean stent expansion was significantly improved following OCT-guided PCI [87.6%±16.6% and 105.8% (IQR, 97.8–119.8%)] as compared with angiography-guided PCI [82.9%±12.9%, P=0.02 and 101.4% (IQR, 91.9–110.2%), P=0.001] and similar to that achieved by IVUS-guidance [86.5%±15.9%, P=0.77 and 106.3% (IQR, 96.7–116.6%), P=0.63]. Untreated dissections and major malapposition were significantly less frequent in OCT group [39 (28%) and 15 (11%)] compared with IVUS group [53/134 (40%), P=0.04 and 28/134 (21%), P=0.02] and angiography group [61 (44%), P=0.006 and 44 (31%), P<0.0001].
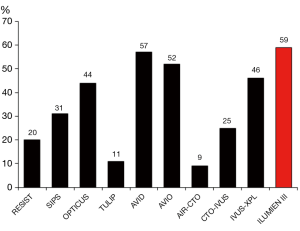
Post-dilatation represents the key corrective measures in the presence of underexpansion or stent strut malapposition and directly affects the primary endpoint of ILUMIEN 3. Post-dilatation was required to achieve a stent expansion of at least 90% in both the proximal and distal halves of the stent relative to the respective reference segment, which represents a so far unique guidance criteria introduced by ILUMIEN 3 investigators. Interestingly, the protocol-mandated expansion target was not achieved in the majority of cases (59%) and the difference in MSA was minimal as compared to the IVUS group, in which no expansion criteria was defined (IVUS 63%). There are two conceivable reasons for this observation: firstly, the expansion target >90% is too ambitious to be achieved even in simple lesions or secondly, the operators did not follow the suggested guidance protocol with sufficient adherence. The article on the study is not providing further insights in this relevant limitation of the study. As in ILUMIEN 3, several other IVUS-guided imaging studies also frequently did not achieve the protocol required expansion goal (Figure 1), albeit guidance-related clinical benefit reportedly emerged (i.e., XPL study). Nevertheless, expansion goals that can be achieved in daily routine appear relevant and insights obtained in ILUMIEN 3 should further assist in determining those.
Regarding the operators’ adherence to the imaging protocol, the higher number of post-dilation performed in the imaging groups (2 vs. 1) and the larger balloons indicate that, the information obtained by imaging was applied at least in part and translated into additional attempts to expand the stent better. Whether a more aggressive approach could have led to a more meaningful difference in stent expansion without compromising safety remains unknown.
Regarding the use of IVUS, no dedicated guidance protocol was available leaving the decision, how IVUS should be used to select stent size and optimize the results at the discretion of the operator. This potentially puts IVUS at disadvantage, at least regarding the primary endpoint measure.
The ILUMIEN 3 study also provides insights into secondary endpoints that were previously associated with adverse events.
Dissections
The frequency of any dissection at the end of the procedure was significantly lower in the OCT (28%) as compared to IVUS (40%, P=0.04) and angiography group (44%, P=0.006). When only considering major dissections (i.e., those with potential clinical impact), the difference between OCT and angiography disappeared, while IVUS was associated with a persisting increased risk. The use of OCT could only lead to fewer dissections by a more frequent identification and subsequent treatment of these tears by additional stent implantation, something that is not mentioned in the article. In the absence of such evidence, the difference is likely explained by chance or alternatively, the use of IVUS could be associated with an increased risk of causing dissections.
Malapposition
Untreated major stent malapposition after PCI was less frequent in the OCT group (11%) as compared with the IVUS (21%, P=0.02) and angiography group (31%, P<0.0001). The findings of malapposed struts are clinically relevant: previous studies consistently reported that malapposition after PCI was the leading cause underlying early and very late stent thrombosis (21,22). In the ILUMIEN 3 trial, major malapposition was defined as ≥200 µm and associated with a stent underexpansion <90%. A previous study reported that struts with an axial malapposition distance of <270 µm will heal spontaneously in 100% of cases (23), suggesting that the axial cutoff for major malapposition may be sensitive. Also, in a previous OCT study in very late stent thrombosis patients, the malapposition length rather than the axial distance emerged as the most relevant correlate of thrombus formation (22). How results between groups would differ when considering a less aggressive threshold for the axial distance (e.g., >300 µm) and when also including the longitudinal extension (e.g., >1 mm) remains open to question.
Stent sizing based on EEM tracing
This study proposed a new stent sizing protocol based on the delineation of the distance between the EEM at the reference vessel segment mainly to overcome the issue encountered in the ILUMIEN 1 study where OCT (as compared to angiography) led the operators select smaller stents (24). The identification of the EEM by OCT may be challenging and time consuming considering that even in the hands of highly trained intracoronary imaging experts, EEM tracing is not possible in one fourth of cases, although similar average stent diameter in the IVUS and OCT group reassuringly confirms that the novel OCT sizing algorithm is resulting in similar stent dimensions. As a simple approach to PCI guidance is a key for the uptake by a broader interventional community, it should be investigated within the ILUMIEN 3 trial data whether a simplified approach considering the mean lumen reference diameter and a standardized upgrading of the stent size could lead to a comparable stent size selection.
Other recent trials with similar focus
Differences in clinical outcomes were not observed. Recently, the results of the OPINION study, a multicenter, prospective, randomized trial testing whether OCT-guided PCI is non-inferior compared with IVUS-guided PCI with respect to the clinical endpoint target vessel failure was presented (25). Indeed, the primary endpoint occurred in a similar frequency between groups (5.2% vs. 4.9%, P for non-inferiority <0.05) confirming non-inferiority of OCT compared with IVUS. In addition, in-stent minimum lumen diameter as assessed by quantitative coronary angiography at 8 months was identical (2.38±0.51 vs. 2.44±0.52 mm, P=0.136). In variance to the ILUMIEN 3, the OPINION study did not include an angiography-guided control arm. Notwithstanding this limitation, the ILUMIEN and OPINION studies consistently provide reassurance that OCT is at least equal to IVUS for PCI guidance, supporting the current shift from IVUS to OCT in most cath labs by important evidence. Although IVUS-guidance has shown to result in improved outcomes in a complex PCI setting, a dedicated outcome study is required with the use of OCT. When designing such a study, lessons learnt should be considered by designing a study that is adequately powered and by considering only patients that indeed are anticipated to benefit from intracoronary imaging guidance, i.e., those with an increased level of complexity on patient (e.g., diabetes mellitus) and lesion level (e.g., long lesions, CTO, left main, and bifurcations). Another challenging task is to define meaningful thresholds based on a distillation of previous studies that investigated stent failures. Thresholds that set the bar for intervention too low ultimately result in overtreatment whereas too high thresholds for corrective measures will leave behind findings that may trigger future cardiovascular events. Finally, simplified criteria represent a key determinant to bring OCT guidance to a success for routine clinical practice in complex PCI.
Acknowledgements
None.
Footnote
Conflicts of Interest: L Räber received speaker fees and grants to the institution from Biotronik, St. Jude Medical/Abbott, Sanofi and Regeneron.
References
- Hong SJ, Kim BK, Shin DH, et al. Effect of intravascular Ultrasound-Guided vs Angiography-Guided Everolimus-Eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA 2015;314:2155-63. [Crossref] [PubMed]
- Kim BK, Shin DH, Hong MK, et al. Clinical Impact of Intravascular Ultrasound-Guided Chronic Total Occlusion Intervention With Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation: Randomized Study. Circ Cardiovasc Interv 2015;8:e002592. [Crossref] [PubMed]
- Tian NL, Gami SK, Ye F, et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention 2015;10:1409-17. [Crossref] [PubMed]
- Kim JS, Kang TS, Mintz GS, et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv 2013;6:369-76. [Crossref] [PubMed]
- Chieffo A, Latib A, Caussin C, et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J 2013;165:65-72. [Crossref] [PubMed]
- Jakabčin J, Spacek R, Bystron M, et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv 2010;75:578-83. [Crossref] [PubMed]
- Russo RJ, Silva PD, Teirstein PS, et al. A randomized controlled trial of angiography versus intravascular ultrasound-directed bare-metal coronary stent placement (the AVID Trial). Circ Cardiovasc Interv 2009;2:113-23. [Crossref] [PubMed]
- Oemrawsingh PV, Mintz GS, Schalij MJ, et al. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP Study). Circulation 2003;107:62-7. [Crossref] [PubMed]
- Mudra H, di Mario C, de Jaegere P, et al. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS Study). Circulation 2001;104:1343-9. [Crossref] [PubMed]
- Frey AW, Hodgson JM, Müller C, et al. Ultrasound-guided strategy for provisional stenting with focal balloon combination catheter: results from the randomized Strategy for Intracoronary Ultrasound-guided PTCA and Stenting (SIPS) trial. Circulation 2000;102:2497-502. [Crossref] [PubMed]
- Schiele F, Meneveau N, Vuillemenot A, et al. Impact of intravascular ultrasound guidance in stent deployment on 6-month restenosis rate: a multicenter, randomized study comparing two strategies--with and without intravascular ultrasound guidance. RESIST Study Group. REStenosis after Ivus guided STenting. J Am Coll Cardiol 1998;32:320-8. [Crossref] [PubMed]
- Stone GW, Sabik JF, Serruys PW, et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med 2016;375:2223-35. [Crossref] [PubMed]
- Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet 2016;388:2743-52. [Crossref] [PubMed]
- Guddeti RR, Matsuo Y, Matsuzawa Y, et al. Clinical implications of intracoronary imaging in cardiac allograft vasculopathy. Circ Cardiovasc Imaging 2015;8:e002636. [Crossref] [PubMed]
- de Jaegere P, Mudra H, Figulla H, et al. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC Study). Eur Heart J 1998;19:1214-23. [Crossref] [PubMed]
- Habara M, Nasu K, Terashima M, et al. Impact of Frequency-Domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance. Circ Cardiovasc Interv 2012;5:193-201. [Crossref] [PubMed]
- Bezerra HG, Attizzani GF, Sirbu VA, et al. Optical coherence tomography versus intravascular ultrasound to evaluate coronary artery disease and percutaneous coronary intervention. JACC Cardiovasc Interv 2013;6:228-36. [Crossref] [PubMed]
- Meneveau N, Souteyrand G, Motreff P, et al. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation 2016;134:906-17. [Crossref] [PubMed]
- Antonsen L, Thayssen P, Maehara A, et al. Optical Coherence Tomography Guided Percutaneous Coronary Intervention With Nobori Stent Implantation in Patients With Non-ST-Segment-Elevation Myocardial Infarction (OCTACS) Trial: Difference in Strut Coverage and Dynamic Malapposition Patterns at 6 Months. Circ Cardiovasc Interv 2015;8:e002446. [Crossref] [PubMed]
- Ali ZA, Maehara A, Genereux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet 2016;388:2618-28. [Crossref] [PubMed]
- Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the National PESTO French registry. Eur Heart J 2016;37:1208-16. [Crossref] [PubMed]
- Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of very late Drug-Eluting stent thrombosis assessed by optical coherence tomography. Circulation 2016;133:650-60. [Crossref] [PubMed]
- Gutiérrez-Chico JL, Wykrzykowska J, Nüesch E, et al. Vascular tissue reaction to acute malapposition in human coronary arteries: sequential assessment with optical coherence tomography. Circ Cardiovasc Interv 2012;5:20-9, S1-8.
- Wijns W, Shite J, Jones MR, et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J 2015;36:3346-55. [Crossref] [PubMed]
- Kubo T, Shinke T, Okamura T, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): Study protocol for a randomized controlled trial. J Cardiol 2016;68:455-60. [Crossref] [PubMed]


