Three-field lymph node dissection in esophageal cancer surgery
Introduction
Although multidisciplinary treatment improved the prognosis of esophageal cancer, it remains recognized as a form of cancer with one of the worse prognosis. Currently, the mainstay of standard treatment for resectable esophageal cancer is a transthoracic esophagectomy with extended lymph node (LN) dissection. Since the transthoracic esophagectomy requires a cervico-thoraco-abdominal approach, the postoperative complication rate remains high. Based on the Japanese national clinical database, the operative mortality following a transthoracic esophagectomy was 3.4%, and the overall morbidity rate was 41.9% (1). Furthermore, since systemic surgical invasiveness is severe during an esophagectomy, the general postoperative condition could decline.
Regarding oncological aspects, the esophagus has abundant lymphatic routes in the submucosal layer and cancerous cell spreads rapidly. Therefore, esophageal cancer can spread easily during early stages of the disease. To regulate the extent of LN metastasis in esophageal cancer, a strategy for extended LN dissection has been established. As one of the standard surgical procedures of an extended LN dissection, three field LN dissection (3FD) was developed in the 1980s’ in Japan and is accepted as a standard treatment throughout the world. In this review, the development of 3FD for esophageal cancer is reviewed, and the oncological benefits and shortcomings are described.
Distribution of LN metastasis in esophageal cancer
Among the gastrointestinal cancers, esophageal cancer exhibits a high incidence rate of LN metastasis even during the early stages of the disease. Furthermore, LN metastasis primarily occurs from the cervix to the abdominal field. Takeuchi et al. reported the mapping of the sentinel LN (SLN), the LNs that are the first to receive lymphatic drainage from a primary tumor site, in patients with superficial esophageal squamous cell carcinoma (ESCC) (2). In the report, 4.7 SLNs existed on average and the location varied from the cervical to abdominal field regardless of the tumor location. In addition, Akutsu et al. reviewed ESCC patients who were recruited to a prospective multi-institutional randomized trial and investigated the distribution of metastatic LN in cT1 esophageal cancer (3). Consequently, upper mediastinal LN metastasis was a frequent site for tumors located in the upper thoracic esophagus (Ut), whereas the abdominal nodes were frequent sites associated with tumors in the lower thoracic esophagus (Lt). However, in the middle thoracic esophagus (Mt), LN metastasis was observed from the cervical field to abdominal field. Tachimori et al. investigated the distribution of LN metastasis in 356 ESCC patients with T1b or T2 disease who underwent a transthoracic esophagectomy with 3FD (4). In the report, in patients with Mt or Lt disease, the incidence of upper mediastinal LN metastasis was frequently observed. Based on those reports, to manage trans-lymphatic metastasis in esophageal cancer, extended LN dissection was recognized as a feasible procedure.
Ideal range of LN dissection in esophageal cancer
Since esophageal cancer metastases occur towards the cervical, thoracic, and abdominal fields even in the early stages of the disease, extended LN dissection appears to be necessary to repress the disease progression. However, an extended LN resection might increase the incidence rate of postoperative complications. Recently, postoperative complications were shown to worsen the prognosis of patients with esophageal cancer (5). Therefore, the ideal range of LN dissection should be assessed before surgery. Based on previous studies, the range of LN dissection can be determined based on the location of the primary tumor, disease progression, histology, and perioperative treatment.
Location of the primary tumor
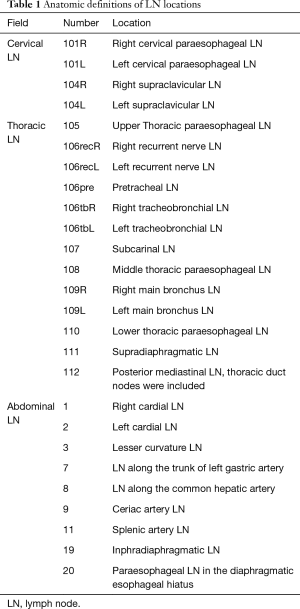
Tachimori et al. reviewed 3,827 ESCC patients using nation-wide registry data in Japan (6). In the study, LN stations adopted by the Japanese Classification of Esophageal Cancer were used (Table 1) (7). Consequently, in ESCC patients with Ut, cervical LN metastasis occurred in 33.4%, perigastric LN metastasis in 9.9%. In the patients with Mt, cervical LN metastasis was observed in 22.8%, perigastric LN metastasis in 27.9%. These results were consistent with the SN mapping. Regarding the efficacy of LN dissection for each LN station, the efficacy index (EI), estimated by the multiplication of the incidence of metastasis and five-year survival rate of patients with LN metastasis for each station, was used (8). In the report, the EI of the cervical node was 14.1 for Ut, 9.2 for Mt, and 5.3 for Lt patients. Regarding the abdominal nodes, the EI was 3.1 for Ut, 9.3 for Mt, and 17.8 for Lt patients. Based on these results, the range of LN dissection should be decided based on the location of the primary tumor. In addition to the thoracic esophagus, there was a difference in the distribution of LN metastasis in cancer of the cervicothoracic junction. Yamasaki et al. reported that cervical and upper mediastinal LN dissection might be sufficient for a cervico centered tumor (9). However, when the center of the tumor is located in the thorax, 3FD may be necessary. Regarding esophageal cancer of the esophagogastric junction, the distance from the EGJ is a useful factor for predicting the incidence of mediastinal LNs (10). Therefore, the location of the primary tumor is a vital factor in determining the range of the LN dissection to treat esophageal cancer.
Full table
Disease progression
Disease progression is one of the important factors to consider when assessing the range of LN dissection. In addition, there is controversy regarding the efficacy of LN dissection around the thoracic duct. Udagawa et al. reported that there were LNs surrounding the thoracic duct and these tissues were identified separately from the LNs around the esophagus. The incident rate of metastasis was 2.2% for pT1b/T2 and 10% for pT3/T4 (11). Matsuda et al. reported that the LNs around the thoracic duct exist from the upper to lower mediastinum and found a correlation between the pN status and the location of LN metastasis around the thoracic duct (12). However, a surgical approach around the thoracic duct might induce chylothorax, which can increase the mortality rate. Furthermore, a thoracic duct resection might influence the postoperative hemodynamic status and efficacy of enteral nutrition (13). In conclusion, the range of LN dissection around the thoracic duct can be determined based on the extent of the tumor progression and patients’ background.
Histology
Yamashita et al. investigated the distribution of LN metastasis in esophagogastric junction cancer, using Japanese nation-wide data (14) and found that the tumor epicenter significantly affected the distribution of LN metastasis. In terms of the correlation between histology and the distribution of LN metastasis, squamous cell carcinoma metastasis occurred towards the upper and middle mediastinum more frequently than adenocarcinoma. Therefore, particularly for cancer of the esophagogastric junction, tumor histology should be considered to determine the range of LN dissection.
Perioperative treatment
Multidisciplinary treatment combining surgery, chemotherapy, and radiotherapy, has been developed for the treatment of esophageal cancer (15,16). Ando et al. conducted the randomized control trial for ESCC patients and showed that preoperative chemotherapy followed by an esophagectomy could extend survival (17). Furthermore, the CROSS trial from the Netherlands evaluated the efficacy of preoperative chemoradiotherapy (18). Consequently, esophageal cancer patients who received chemoradiotherapy followed by surgery exhibited a longer overall survival than those who underwent surgery alone. Currently, perioperative chemotherapy or chemoradiotherapy are accepted as standard treatments throughout the world. Furthermore, patients who refused to undergo an esophagectomy selected definitive chemoradiotherapy, in which the radiation dose exceeds 50 Gy, as an alternative. Additionally, when the tumor persisted, a salvage esophagectomy could be performed following definitive chemoradiotherapy. Tachimori et al. reported that the incidence of postoperative complications could increase following a salvage esophagectomy (19). Therefore, to preserve the blood flow to the trachea and maintain respiratory function, the range of LN dissection can be reduced. Therefore, the intensity of perioperative might influence the indication for 3FD.
Definition and establishment of 3FD
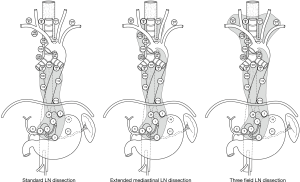
3FD in esophagectomy is defined as a procedure for cervico-thoraco-abdominal LN dissection. In Japan, a transthoracic esophagectomy with 3FD is recommended to be performed in accordance with the Japanese guidelines for the diagnosis and treatment of esophageal cancer. In the cervix, supraclavicular LN (No. 104) and paracervical esophageal nodes (No. 101) need to be dissected. In a thoracic lymphadenectomy, LNs around the bilateral recurrent laryngeal nerve (No. 106recR, 106recL, and 106tbL), paraesophageal LN (No. 105, 108, and 110), paratracheal LN (No. 107 and 109), posterior mediastinal LN (No. 112), and supradiaphragmatic LN (No. 111) are included in routine dissection. The dissection of LNs around the thoracic duct should be determined based on the location of the primary tumor and disease progression. In the abdominal field, paracardial LN (No. 1, 2), LNs along the lesser curvature (No. 3), LNs along the trunk of the left gastric artery (No. 7), LNs around the abdominal esophagus (No. 20), and inphradiaphragmatic LNs (No. 19) are dissected.
The surgical procedure for a lymphadenectomy for esophageal cancer has been established worldwide. Since Torek reported the first case of a successful esophagectomy in 1913 (20), the safety and efficacy of an esophagectomy for esophageal cancer have been reported, and the range of LN dissection has been extended. Fujita reviewed the history of LN dissection in esophageal cancer and reported that 3FD was initiated by two Japanese Surgeons, Kajitani and Sannohe (21,22). Kajitani initiated the systematic LN dissection of LNs around the recurrent laryngeal nerve, developing the upper mediastinal LN dissection. Subsequently, Sannohe reported cervical LN dissection and the incidence of metastasis in patients who underwent 3FD. After these reports, the safety and survival benefits of 3FD were shown in Japan (23-31). In 1990’s, 3FD was accepted worldwide and safely performed (32-34). Moreover, Altorki et al. reported 80 patients who underwent an esophagectomy with 3FD, of which 30% of the participants were upstaged following 3FD (32). Lerut et al. reviewed 192 patients with 3FD and revealed acceptable safety and a hospital mortality rate of 1.2% (33). Based on these previous reports, an esophagectomy with 3FD is accepted as a currently acceptable procedure.
When we discuss the LN dissection of transthoracic esophagectomy, the dissection in the upper mediastinum must be highlighted. Although the upper mediastinal LN dissection was included in both 2FD and 3FD, the surgical procedure and intensity were historically different between the two, depending on the patients’ background, tumor characteristics, and countries. As shown above, the range of LN metastasis could be related to tumor histology. Even if the tumor is located in the same region, ESCC tends to metastasis towards the upper mediastinum (14,35). Furthermore, ESCC is common in Asia and South America, while the incidence rate of adenocarcinoma is higher in North America and Europe (36,37). Based on such background variability, upper mediastinal LN dissection is particularly focused in the region where patients frequently suffer from ESCC, including Japan. Fujita reviewed the variation of LN dissection in transthoracic esophagectomy for esophageal cancer and classified it into standard dissection, extended dissection, and 3FD (28,29); according to the report, for standard LN dissection, the dissection in the upper mediastinum can be omitted (Figure 1).
Surgical procedure of 3FD
In 3FD for esophageal cancer, LNs from the cervix to abdomen are routinely dissected. Particularly in an upper mediastinal LNs dissection, LNs around the bilateral recurrent laryngeal nerve (No. 106recR and 106recL) contributed to the efficacy since the incidence rate of LN metastasis to that field is high. However, radical dissection in that field might induce postoperative recurrent laryngeal nerve paralysis. Recently, a thoracoscopic esophagectomy has been accepted worldwide, and long-term survival is currently under investigation (38). Through the introduction of a thoracoscopic esophagectomy, more precise surgical procedures with a magnified view have been achieved.
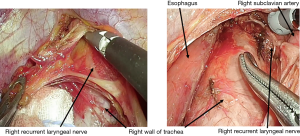
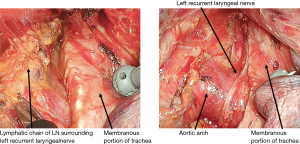
In the thoracoscopic esophagectomy, the esophagus is initially mobilized from the dorsal to the ventral side. When the thoracic duct is resected simultaneously, the aortic arch and left pleura are identified, and the left subclavian artery is confirmed. When a No. 106recR dissection is initiated, the right vagus nerve is traced cranially, and the caudal edge of the right subclavian artery is identified. The tissue which includes the No. 106recR LN is mobilized from the bronchus, and the right recurrent laryngeal nerve is identified. When the right recurrent laryngeal nerve is confirmed, the plane on the surface of the nerve is traced toward the cervix. Dissecting the esophageal branch of the right recurrent laryngeal nerve, the No. 106recR is removed (Figure 2). In the dissection of No. 106recL, the esophagus is elevated using the double taping method. Dissecting the tissue on the left side of the trachea, the lymphatic chain which includes the No. 106recL is mobilized towards the esophagus from No. 106pre. Subsequently, the left recurrent laryngeal nerve is identified in the lymphatic chain, and the surrounding tissue is dissected preserving left recurrent laryngeal nerve (Figure 3). When the cervical approach is added, the bilateral recurrent laryngeal nerve is identified, and the bilateral No. 101 can be dissected.
Safety and efficacy of 3FD and comparison with 2FD
To date, an esophagectomy with 3FD is recognized as a standard treatment. There are reasons for the extended postoperative survival associated with 3FD. First, extended LN dissections (e.g., 3FD) can increase the radicality in an esophagectomy. As shown above, esophageal cancer can lead to LN metastasis from the cervical to the abdominal fields, and an extended range of LN dissection (e.g., 3FD) can lead to the elimination of tumor cells. Second, an extended LN dissection increases the accuracy of staging, leading to an improvement in the postoperative survival for each stage classification. In addition, Baba et al. reported that the number of negative LNs correlated with survival in ESCC patients (39). In another study, a higher number of negative LNs were shown to be correlated with improved postoperative survival. Even if an upper mediastinal LN dissection could be performed both in 3FD and 2FD, the number of dissected LNs in the upper mediastinum can be greater for 3FD due to the addition of the cervical approach. Therefore, 3FD may be beneficial for postoperative survival. In contrast, since postoperative complications might be induced in 3FD, leading to a poor prognosis and decreased quality of life, Nozoe et al. insisted that 2FD was sufficient for T1b ESCC (40). Furthermore, Wong et al. compared 2FD with 3FD using a prospective database, and described that there were no survival benefits in the 3FD group (41).
Recently, several systematic reviews and meta-analyses have been reported (42-44). Shang et al. analyzed the long-term survival and showed that 3FD was superior to 2FD for patients with LN metastasis in the cervical or upper mediastinal LNs (44). Ma et al. reported a meta-analysis and demonstrated that 3FD could lead to improved survival rates following an esophagectomy (43). The only published randomized control trial was conducted by Nishihira et al. (45) in which esophageal cancer patients were randomly classified into conventional LN dissection and 3FD groups. In the safety profile, recurrent laryngeal nerve palsy tended to be increased in the conventional LN dissection group (30%) compared to the 3FD group (56%). In terms of the postoperative survival, the 5-year survival rate was 48% for the patients that received a conventional LN dissection, and 66% in the 3FD group without significance. Although this is the only randomized trial and was vital for investigating the clinical significance of 3FD in esophageal cancer, the sample size might not be sufficient to clarify the statistical difference.
There are several retrospective comparisons which have investigated the prognosis associated with 3FD (25,26,46-49). As shown in Table 2, the majority of studies showed a superiority of 3FD. However, most of the studies were reported in the 1990’s, and the patient selection was not defined before surgery. In some studies, only patients with good surgical outcomes were included. Furthermore, in 2010, Shim et al. reported that there was no significant difference between 2FD and 3FD (49). As Ando et al. indicated, the survival of esophageal cancer patients improved due to advancements in surgical techniques and perioperative management in the 1990’s (27). In addition, regarding upper mediastinal LN dissections, the indication and radicality might differ between the 2FD and 3FD groups. Therefore, the evidence suggesting that 3FD is superior to 2FD remains immature. In a number of previous reviews, the prospective randomized trial was described as a demanding step. However, if the indication of an upper mediastinal LN dissection is verified and performed in both groups, the superiority of 3FD might be minimized for several types of esophageal cancer.
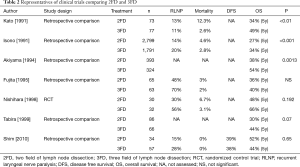
Full table
Shortcoming of 3FD
An esophagectomy with 3FD might increase postoperative complications. In particular, following an LN dissection surrounding the bilateral recurrent laryngeal nerve and supraclavicular LNs, swallowing function has been found to decrease. Yasuda et al. compared patients with 3FD to 2FD and reported that laryngeal elevation was insufficient in a patient who underwent 3FD (50). Consequently, the incidence of aspiration increased. Moreover, Nakamura et al. investigated the severity of gastrointestinal dysfunction between 2FD and 3FD and showed that an insufficiency in the gastrointestinal tract (e.g., decreased physical activity, reflux, and passage dysfunction) was significantly increased in patients who had undergone 3FD (51). In China, recurrent laryngeal nerve palsy frequently occurs in patients following 3FD (34). Recently, postoperative pneumonia has to be associated with a poor prognosis (5), and the safety and efficacy of LN dissection need to be balanced.
Future perspectives
Transthoracic esophagectomy with 3FD might be beneficial for patient prognosis but shortcomings, including postoperative complications and decreased gastrointestinal function exist. Furthermore, previous evidence has been reported by high volume centers in which the surgical team had sufficient experience to manage esophageal cancer patients. However, an esophagectomy can be performed in community hospitals in various countries. Therefore, surgical procedures should be standardized further, and safety should be maintained.
To avoid an esophagectomy, chemoradiotherapy could be an alternative form of treatment. Previously, chemoradiotherapy was found to be inferior to surgery, and radiation-related adverse events could occur. However, the radiation protocol has improved, and radiotherapy could be a sufficient alternative to esophagectomy. Regarding the omission of the 3FD, Shiozaki et al. reported that ESCC patients without metastasis in No. 106recR and 106recL could avoid cervical LN dissection in Mt or Lt esophageal cancer (52,53). Therefore, while we pursue the safety and efficacy of surgical procedures, minimally invasive approaches can be achieved. Further improvement of multidisciplinary treatment is necessary to extend patient postoperative survival. Recently, the efficacy of intense perioperative treatment in esophageal cancer has been reported, and the significance of minimally invasive surgical procedure is undergoing verification (18,38). The ideal combination of perioperative treatment and feasible surgery must be established to improve the oncological outcome of esophageal cancer patients further.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259-66. [Crossref] [PubMed]
- Takeuchi H, Fujii H, Ando N, et al. Validation study of radio-guided sentinel lymph node navigation in esophageal cancer. Ann Surg 2009;249:757-63. [Crossref] [PubMed]
- Akutsu Y, Kato K, Igaki H, et al. The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: From the results of JCOG0502, a prospective multicenter study. Ann Surg 2016;264:1009-15. [Crossref] [PubMed]
- Tachimori Y, Nagai Y, Kanamori N, et al. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus 2011;24:33-8. [Crossref] [PubMed]
- Booka E, Takeuchi H, Nishi T, et al. The impact of postoperative complications on survivals after esophagectomy for esophageal cancer. Medicine 2015;94:e1369. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 10th edition: part I. Esophagus 2009;6:1-25.
- Udagawa H, Ueno M, Shinohara H, et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 2012;106:742-7. [Crossref] [PubMed]
- Yamasaki M, Miyata H, Miyazaki Y, et al. Pattern of lymphatic spread of esophageal cancer at the cervicothoracic junction based on the tumor location: Surgical treatment of esophageal squamous cell carcinoma of the cervicothoracic junction. Ann Surg Oncol 2015;22:S750-7. [Crossref] [PubMed]
- Kurokawa Y, Hiki N, Yoshikawa T, et al. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery 2015;157:551-5. [Crossref] [PubMed]
- Udagawa H, Ueno M, Shinohara H, et al. Shoud lymph nodes along the thoracic duct be dissected routinely in radical esophagectomy? Esophagus 2014;11:204-10. [Crossref]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Clinical outcome of transthoracic esophagectomy with thoracic duct resection: Number of dissected lymph node and distribution of lymph node metastasis around the thoracic duct. Medicine (Baltimore) 2016;95:e3839. [Crossref] [PubMed]
- Aiko S, Yoshizumi Y, Matsuyama T, et al. Influences of thoracic duct blockage on early enteral nutrition for patients who underwent esophageal cancer surgery. Jpn J Thorac Cardiovasc Surg 2003;51:263-71. [Crossref] [PubMed]
- Yamashita H, Seto Y, Sano T, et al. Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer 2017;20:69-83. [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Current Advancement in multidisciplinary treatment for resectable cStage II/III esophageal squamous cell carcinoma in Japan. Ann Thorac Cardiovasc Surg 2016;22:275-83. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Tachimori Y, Kanamori N, Uemura N, et al. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2009;137:49-54. [Crossref] [PubMed]
- Torek F. The first successful case of resection of the thoracic portion of the oesophagus for carcinoma. Surg Gynecol Obstet 1913;16:614-7.
- Fujita H. History of lymphadenectomy for esophageal cancer and the future prospects for esophageal cancer surgery. Surg Today 2015;45:140-9. [Crossref] [PubMed]
- Sannohe Y, Hiratsuka R, Doki K. Lymph node metastases in cancer of the thoracic esophagus. Am J Surg 1981;141:216-8. [Crossref] [PubMed]
- Kato H, Tachimori Y, Mizobuchi S, et al. Cervical, mediastinal, and abdominal lymph node dissection (three-field dissection) for superficial carcinoma of the thoracic esophagus. Cancer 1993;72:2879-82. [Crossref] [PubMed]
- Baba M, Aikou T, Yoshinaka H, et al. Long-term results of subtotal esophagectomy with three-field lymphadenectomy for carcinoma of the thoracic esophagus. Ann Surg 1994;219:310-6. [Crossref] [PubMed]
- Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 1995;222:654-62. [Crossref] [PubMed]
- Tabira Y, Kitamura N, Yoshioka M, et al. Significance of three-field lymphadenectomy for carcinoma of the thoracic esophagus based on depth of tumor infiltration, lymph nodal involvement and survival rate. J Cardiovasc Surg (Torino) 1999;40:737-40. [PubMed]
- Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg 2000;232:225-32. [Crossref] [PubMed]
- Fujita H, Sueyoshi S, Tanaka T, et al. Optimal lymphadenectomy for squamous cell carcinoma in the thoracic esophagus: Comparing the short- and long-term outcome among the four types of lymphadenectomy. World J Surg 2003;27:571-9. [Crossref] [PubMed]
- Kakegawa T, Fujita H. A History of esophageal surgery in the twentieth century. Gen Thorac Cardiovasc Surg 2009;57:55-63. [Crossref] [PubMed]
- Tachibana M, Kinugasa S, Yoshimura H, et al. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. Am J Surg 2005;189:98-109. [Crossref] [PubMed]
- Natsugoe S, Matsumoto M, Okumura H, et al. Clinical course and outcome after esophagectomy with three-field lymphadenectomy in esophageal cancer. Langenbecks Arch Surg 2010;395:341-6. [Crossref] [PubMed]
- Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. [Crossref] [PubMed]
- Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962-72. [Crossref] [PubMed]
- Fang WT, Chen WH, Chen Y, et al. Selective three-field lymphadenectomy for thoracic esophageal squamous carcinoma. Dis Esophagus 2007;20:206-11. [Crossref] [PubMed]
- Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005;242:566-73. [PubMed]
- Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 2008;100:1184-7. [Crossref] [PubMed]
- Dikken JL, Lemmens VE, Wouters MW, et al. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur J Cancer 2012;48:1624-32. [Crossref] [PubMed]
- Kataoka K, Takeuchi H, Mizusawa J, et al. A randomized Phase III trial of thoracoscopic versus open esophagectomy for thoracic esophageal cancer: Japan clinical oncology group study JCOG1409. Jpn J Clin Oncol 2016;46:174-7. [Crossref] [PubMed]
- Baba Y, Watanabe M, Shigaki H, et al. Negative lymph-node count is associated with survival in patients with resected esophageal squamous cell carcinoma. Surgery 2013;153:234-41. [PubMed]
- Nozoe T, Kakeji Y, Baba H, et al. Two-field lymph-node dissection may be enough to treat patients with submucosal squamous cell carcinoma of the thoracic esophagus. Dis Esophagus 2005;18:226-9. [Crossref] [PubMed]
- Wong J, Weber J, Almhanna K, et al. Extent of lymphadenectomy does not predict survival in patients treated with primary esophagectomy. J Gastrointest Surg 2013;17:1562-8. [Crossref] [PubMed]
- Ye T, Sun Y, Zhang Y, et al. Three-field or two-field resection for thoracic esophageal cancer: a meta-analysis. Ann Thorac Surg 2013;96:1933-41. [Crossref] [PubMed]
- Ma GW, Situ DR, Ma QL, et al. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol 2014;20:18022-30. [PubMed]
- Shang QX, Chen LQ, Hu WP, et al. Three-field lymph node dissection in treating the esophageal cancer. J Thorac Dis 2016;8:E1136-49. [Crossref] [PubMed]
- Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 1998;175:47-51. [Crossref] [PubMed]
- Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991;48:411-20. [Crossref] [PubMed]
- Kato H, Watanabe H, Tachimori Y, et al. Evaluation of neck lymph node dissection for thoracic esophageal carcinoma. Ann Thorac Surg 1991;51:931-5. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72. [Crossref] [PubMed]
- Shim YM, Kim HK, Kim K. Comparison of survival and recurrence pattern between two-field and three-field lymph node dissections for upper thoracic esophageal squamous cell carcinoma. J Thorac Oncol 2010;5:707-12. [Crossref] [PubMed]
- Yasuda T, Yano M, Miyata H, et al. Evaluation of dysphagia and diminished airway protection after three-field esophagectomy and a remedy. World J Surg 2013;37:416-23. [Crossref] [PubMed]
- Nakamura M, Kido Y, Hosoya Y, et al. Postoperative gastrointestinal dysfunction after 2-field versus 3-field lymph node dissection in patients with esophageal cancer. Surg Today 2007;37:379-82. [Crossref] [PubMed]
- Li H, Yang S, Zhang Y, et al. Thoracic recurrent laryngeal lymph node metastases predict cervical node metastases and benefit from three-field dissection in selected patients with thoracic esophageal squamous cell carcinoma. J Surg Oncol 2012;105:548-52. [Crossref] [PubMed]
- Shiozaki H, Yano M, Tsujinaka T, et al. Lymph node metastasis along the recurrent nerve chain is an indication for cervical lymph node dissection in thoracic esophageal cancer. Dis Esophagus 2001;14:191-6. [Crossref] [PubMed]