No prophylaxis is non-inferior and cost-saving to prophylactic intravenous hydration in preventing contrast-induced nephropathy on requiring iodinated contrast material administration
In the 1980s, Eisenberg et al. demonstrated that the development of contrast-induced nephropathy (CIN) in patients with chronic kidney disease (CKD) undergoing contrast-enhanced examination may be prevented by intravenous administration of physiological saline during the examination (1,2). Trivedi et al. conducted a randomized prospective trial to assess the role of saline hydration on the development of CIN (3). A total of 53 patients with normal renal function who were going to undergo nonemergency cardiac catheterization were randomized to a group of patients receiving normal saline intravenously or a group of patients allowed unrestricted oral fluids. CIN developed in 1 of the 27 patients (3.7%) receiving saline infusion and 9 of the 26 patients (34.6%) with unrestricted oral fluids (P=0.005), indicating that saline hydration significantly decreases the incidence of CIN. According to these findings, it is recommended that patients receive intravenous solutions such as physiological saline prior to contrast exposure to prevent CIN. However, clinical-effectiveness and cost-effectiveness of this prophylactic hydration treatment in protecting renal function has not been adequately studied in the population targeted by the guidelines, against a group receiving no prophylaxis.
Recently, Nijssen and colleagues conducted a prospective, randomised, phase 3, parallel-group, open-label, non-inferiority trial (AMACING) of patients at risk of CIN according to current guidelines (4). High-risk patients [with an estimated glomerular filtration rate (eGFR)] of 30–59 mL/min/1.73 m2] undergoing an elective procedure requiring iodinated contrast material administration were randomly assigned (1:1) to receive intravenous 0.9% NaCl or no prophylaxis. The primary outcome was incidence of CIN, defined as an increase in serum creatinine (SCr) from baseline of more than 25% or 44 µmol/L within 2–6 days of contrast exposure, and cost-effectiveness of no prophylaxis compared with intravenous hydration in the prevention of CIN. A total of 660 consecutive patients were randomly assigned to receive no prophylaxis (n=332) or intravenous hydration (n=328). CIN was recorded in 8 (2.6%) of 307 non-hydrated patients and in 8 (2.7%) of 296 hydrated patients. The absolute difference (no hydration vs. hydration) was −0.10% (one-sided 95% CI, −2.25 to 2.06; one-tailed P=0.4710). No hydration was cost-saving relative to hydration. No haemodialysis or related deaths occurred within 35 days, and 18 (5.5%) of 328 patients had complications associated with intravenous hydration. They found no prophylaxis to be non-inferior and cost-saving in preventing CIN compared with intravenous hydration according to current clinical practice guidelines.
These results have important investigative implications. Many clinical trials of how to prevent CIN have been done, but no randomised trial has prospectively compared intravenous hydration as proposed by the guidelines to no prophylaxis in the high-risk population targeted by the guidelines. CIN incidences found in the AMACING trial were low (2.6–2.7%), and no haemodialysis or related deaths occurred within 35 days. The AMACING study found no prophylaxis to be non-inferior to prophylactic intravenous hydration in the prevention of CIN, as well as cost-saving. We often have worried about the development of CIN and subsequent haemodialysis in CKD patients on use of iodinated contrast material administration (5). Based on these results, withholding prophylaxis for high-risk patients with eGFR higher than 29 mL/min/1.73 m2 might be considered without compromising patient safety.
On the other hand, this study excluded the patients with eGFR lower than 30 mL/min/1.73 m2, emergencies and intensive care patients with higher contrast volume or haemodynamic instability. Although the difference in risk of CIN between hydrated and non-hydrated groups is small within the subgroup analysis regarding interventional versus diagnostic procedure, these results cannot be generalised to include such patients with acute myocardial infarction where some benefit of hydration has been found (6,7). In addition, patients undergoing emergent percutaneous coronary intervention (PCI) are at high risk for CIN because of hemodynamic instability and infeasibility of adequate prophylaxis (8,9). There were only three clinical trials on the prevention of CIN including a randomly assigned group not receiving prophylaxis (Table 1) (6,7,10). Two were done in patients with ST-elevation myocardial infarction, most of whom had normal renal function, and both found prophylaxis superior which might be explained by other factors such as higher contrast volume, haemodynamic instability and nephrotoxic treatments inherent to this population (6,7). James et al. performed a large meta-analysis and systematic review of mortality associated with CIN (11). They found that after adjustment for variables other than CIN that could contribute to the mortality, there was a significant reduction in the association between CIN and mortality.
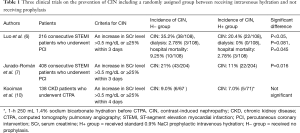
Full table
In the future, large clinical trials will provide further insight into the potential strengths of these results whether giving no prophylaxis is non-inferior to standard care prophylactic hydration on undergoing an elective procedure requiring iodinated contrast material administration.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Eisenberg RL, Bank WO, Hedgcock MW. Renal failure after major angiography. Am J Med 1980;68:43-6. [Crossref] [PubMed]
- Eisenberg RL, Bank WO, Hedgock MW. Renal failure after major angiography can be avoided with hydration. AJR Am J Roentgenol 1981;136:859-61. [Crossref] [PubMed]
- Trivedi HS, Moore H, Nasr S, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract 2003;93:C29-34. [Crossref] [PubMed]
- Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet 2017;389:1312-22. [Crossref] [PubMed]
- Sato A, Aonuma K, Watanabe M, et al. Association of contrast-induced nephropathy with risk of adverse clinical outcomes in patients with cardiac catheterization: From the CINC-J study. Int J Cardiol 2017;227:424-9. [Crossref] [PubMed]
- Luo Y, Wang X, Ye Z, et al. Remedial hydration reduces the incidence of contrast-induced nephropathy and short-term adverse events in patients with ST-segment elevation myocardial infarction: a single-center, randomized trial. Intern Med 2014;53:2265-72. [Crossref] [PubMed]
- Jurado-Román A, Hernández-Hernández F, García-Tejada J, et al. Role of hydration in contrast-induced nephropathy in patients who underwent primary percutaneous coronary intervention. Am J Cardiol 2015;115:1174-8. [Crossref] [PubMed]
- Abe D, Sato A, Hoshi T, et al. Clinical predictors of contrast-induced acute kidney injury in patients undergoing emergency versus elective percutaneous coronary intervention. Circ J 2014;78:85-91. [Crossref] [PubMed]
- Watabe H, Sato A, Hoshi T, et al. Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. Int J Cardiol 2014;174:57-63. [Crossref] [PubMed]
- Kooiman J, Sijpkens YW, van Buren M, et al. Randomised trial of no hydration vs. sodium bicarbonate hydration in patients with chronic kidney disease undergoing acute computed tomography-pulmonary angiography. J Thromb Haemost 2014;12:1658-66. [Crossref] [PubMed]
- James MT, Samuel SM, Manning MA, et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv 2013;6:37-43. [Crossref] [PubMed]


