Treatment for sternoclavicular joint infections: a multi-institutional study
Introduction
The sternoclavicular joint (SCJ) is composed of the inferior portion of the medial head of the clavicle, the outer upper aspect of the manubrium and the cartilage of the first rib, with the great vessels lying beneath it (1). A SCJ infection is a rare complication that has been associated with comorbidities; such as diabetes mellitus (DM), intravenous drug abuse, rheumatoid arthritis, and immunosuppressive disorders (2). The incidence of septic arthritis in the SCJ has been reported to be approximately 1% of all infectious arthritis cases in the general population (3). Due to the rarity of this disease, the management of SCJ infections is controversial and can range from conservative antibiotic therapy to radical surgery including resection of the SCJ (4). Secondary to the joint being in close proximity to the great vessels (aorta and superior vena cava) and the lack of sufficient tissue to help with wound healing, management often presents a challenge and thoracic surgeons are consulted (5). In addition, to surgical intervention, imaging modalities have been employed to better evaluate the severity of disease and assist with surgical planning. A delay in diagnosis may lead to severe complications, including: empyema, mediastinitis and/or osteomyelitis. These complications emphasize the significance and benefit to the patient for an early diagnosis (4).
Due to the lack of consensus for treatment strategies, we evaluated our experience with these infections and developed a treatment algorithm that we use and others can use to best ensure an early diagnosis and treatment for SCJ infections.
Methods
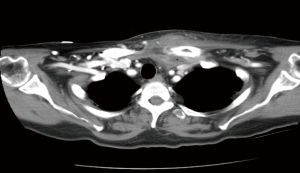
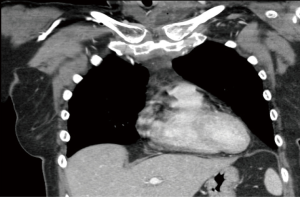
A retrospective chart review approved by the Loma Linda University and Loma Linda Veterans Administration (VA) Institutional Review Board Review (IRB) was conducted of all patients who underwent treatment of SCJ infections at the university medical center and the VA hospital between 2001 and 2014. Patients’ medical records were reviewed, including: past medical history, laboratory results, imaging, treatments and functionality on follow up. Complete blood cell count (CBC) specifically evaluating the white blood cell count (WBC), sodium, creatinine, glucose and hemoglobin A1c were reviewed. In addition, patient charts were assessed for presenting signs and symptoms including pain at the joint, erythema at the joint, drainage, fever greater than 38.3 °C, tachycardia (heart rate greater than 100) and blood pressure, specifically noting if patients were hypotensive (systolic blood pressure less than 90 mmHg). All patients underwent imaging which consisted of computed tomography (CT) scan of the chest (Figures 1 and 2). Patients’ records were also reviewed for whether patient was transferred from an outside facility, as well as diagnosis at the outside facility. The hospital course consisted of preoperative treatment, the type of operation, findings at the time of the operation, laterality and antimicrobial therapy. The time from when thoracic surgery was consulted until when the patient went to the operating room, wound cultures and return to the operating room were also noted. Furthermore, whether patients were placed on antibiotics prior to surgery, if blood cultures were obtained and the results were additional data points that were recorded. Patients charts were further reviewed after they had surgery for functionality of the extremities, the length of physical therapy after surgery, the need for home intravenous antibiotics and the length of follow up.
Results
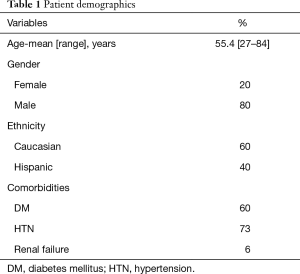
All fifteen patients were diagnosed with an infection of the SCJ and underwent treatment. The patient demographics are described in Table 1. Three patients were female and twelve were male. Nine were found to be Caucasian and six were Hispanic. Patients’ underlying medical problems consisted of hypertension (HTN) [11], DM [9], and renal failure [1]. Of the 15 patients, none were immunosuppressed and none were known intravenous drug users.
Full table
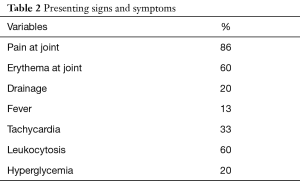
On physical examination, 13 patients reported pain at the joint. Nine presented with erythema at the joint. Three were found to have drainage from skin overlying the joint. Two were febrile between 38.3 and 41 degrees Celsius. Five had tachycardia (100–135 beats per minute). Nine patients were hypertensive with blood pressure greater than 140 systolic and one was hypotensive with systolic pressure less than 90 mmHg (Table 2). The mean duration from admission to when the patient was taken to the operating room was 5.8 days (range, 1–30 days). Of the eight patients who were transferred from an outside facility, only three were transferred with a concern for a possible abscess. All of the patients had a CT scan of the chest, demonstrating a disease process ranging from a simple enhanced attenuation in the SCJ region suggesting a fluid collection, to osteomyelitis. Nine patients were found to have the right SCJ involved and six had an infection on the left. On laboratory examination, nine were found to have leukocytosis and three were hyperglycemic with the median blood sugar 321 and range from 244–370 mg/dL. One patient was thrombocytopenic and one patient was hyponatremic with sodium level of 123 mmol/L. Preoperative blood cultures were obtained from twelve patients of which eight grew out S. aureus and all fifteen were started on intravenous antibiotics, ten on vancomycin, one on each of: clindamycin, piperacillin/tazobactam, penicillin, ampicillin/sulbactam and cefuroxime. The surgical management for these patients consisted of all patients having incision, drainage and joint resection, and three patients underwent pectoralis major flap reconstruction. The surgeons debrided the infection to healthy tissue. After the SCJ was resected with the oscillating saw, the muscle and fascia were debrided to health tissue. All pockets were probed and all purulent drainage was released. Intra-operatively seven patients were found to have osteomyelitis. For these seven patients, further returns to the operating room for washout and debridement were required. The seven patients were taken back to the operating room for a persistent infection and required a second operation to obtain source control. On each return to the operating room, the surgeon made sure to release all purulent drainage, and debride to the healthiest muscle layer, to ensure good wound healing. The average time to return to the operating room was 4.1 days. The patients who required a pectoralis muscle flap had large vacant spaces that would leave the aorta and/superior vena cava at risk to exposure. For the three patients that underwent a flap the average size of the defect was 8 cm. Therefore, it was decided since those sites were significantly larger and would not heal without complete coverage of the underlying vasculature, a pectoralis muscle flap would be the best to ensure complete coverage.
Full table
An organism was identified in all SCJ infections from cultures taken from the surgical specimen. Eleven patients grew staphylococcus species, propionibacterium acnes [1], bacteroides fragilis [1] and streptococcus [1]. All patients had normalization of the WBC count and the average time to resolution was 3.6 days.
The average length of hospital stay was 19 days. One patient died, due to complications of sepsis. Of the remaining patients, one died after discharge due to unknown causes. All fourteen patients were discharged on intravenous antibiotics. Three patients were discharged with negative pressure wound therapy, eight patients worked with physical therapy while in the hospital and three were discharged with outpatient physical therapy.
The time to follow-up ranged from 1 month up to 11 months. On follow up visits, three patients continued to work with physical therapy, four stated they experienced limitations such as pain in the region of surgery, inability to lift heavy objects and decreased range of motion of the joint. All surviving patients had resolution of their SCJ infection.
Discussion
SCJ infections are a rare disease associated with high morbidity highlighting the importance of developing treatment protocols to ensure positive outcomes. Often patients are medically managed with no improved response to antibiotics necessitating surgical management, which in itself, has its own challenges due to the joint’s proximity to the great vessels (5).
Ross et al. (3) reported 180 cases of SCJ infections of which he reports associated risk factors including intravenous drug use, DM, infected central lines, renal failure (creatinine greater than 1.5 mg/dL), cardiac disease, immunosuppressive disorders, rheumatoid arthritis and trauma. The associated risks in this study were found to be: DM in 60% of patients, renal failure in 6% and HTN in 73%. Several case reports have identified SCJ infections in healthy adults without any of the risk factors mentioned above (6,7); thus, making it important to have SCJ infections as part of the differential diagnosis in any patient who presents with swelling surrounding the joint, a painful joint, erythema or drainage from that region (8).
Carlos et al. (9) described nine patients with SCJ infections of which 77% underwent CT imaging to assess the extent of the disease, which is consistent with our findings in which all patients underwent CT scan as part of their evaluation. Although in this experience all patients had CT scans to evaluate the disease, MRI can also serve as an appropriate modality to assess the extent of the disease (9).
In this study 80% of the patients had blood cultures drawn prior to surgery, of which 47% were identified to be staphylococcus aureus. Intraoperative cultures were obtained in all patients. Seventy-three percent (73%) were found to be staphylococcus aureus, 6.7% bacteroides fragilis, 6.7% staphylococcus epidermidis, 6.7% propionibacterium acnes and 6.7% streptococcus. Burkhart et al. (4) reported similar results with all the patients having had wound cultures taken during surgery and 65% of the patients were found to have S. aureus as the source of infection. The treatment strategy of Burkhart et al was; prolonged intravenous antibiotics similar to these study results. All surviving patients were discharged home with intravenous antibiotics in this study (8,10).
Vancomycin was started for methicillin resistant staphylococcus aureus (MRSA) infections. This was started in ten patients prior to final sensitivities and then deescalated to specific organisms, consistent with the recommendations of Brook et al. (10), which patients should be started on an antibiotic to cover any possible MRSA infections due to the increased prevalence in the community. Usually vancomycin is drug of choice, but other antibiotics such as daptomycin, linezolid, quinupristin/dalfopristin are also effective for treating MRSA infections (3).
Puri et al. (11) reported that twenty patients with SCJ infections were treated with surgical intervention. Ten patients underwent drainage and debridement with an open wound with delayed wound healing. The other ten patients had a joint resection and pectoralis major flap closure. They reported complete wound healing in all their patients without any restrictions on physical activity.
Carlos et al. (9) describes their experience with SCJ infections and report eight patients who underwent surgical treatment. Four patients had a drainage and debridement, with the remaining four having an en bloc resection of the joint with the ipsilateral pectoralis major muscle flap. They reported complete wound healing in all their patients and no restrictions in any of the patients including those who had a joint resection.
Song et al. (5) reported their experience with seven patients in which six underwent incision, drainage and debridement with antibiotic therapy, with recurrence of disease even after surgery, thus concluding that this procedure is ineffective. These patients required more aggressive surgical therapy consisting of resection of the joint with ipsilateral pectoralis major muscle flap as they had recurrence after going medical therapy with drainage and debridement. They all had complete wound healing with no wound complications or any functional impairment in the upper extremity.
Burkhart et al. (4) reported performing incision, drainage and debridement in six patients and joint resection in twenty patients. Six patients who underwent drainage and debridement had recurrence of disease and required joint resection. They reported complete wound healing in all their patients who are still alive, as one died due to complications of sepsis. Their patients reported no limitations in functionality of the upper extremity.
Conclusions
Fifteen patients were identified in this series, of which all underwent surgery: Twelve patients had a joint resection with intravenous antibiotic therapy and three had a joint resection with ipsilateral pectoralis major muscle flap and intravenous antibiotics. One patient died secondary to complications from sepsis and a second patient, died after discharge due to unknown causes. Despite surgical intervention all of the patients that were discharged required prolonged antibiotics and only three reported limitations including; pain at the joint, an inability to lift heavy objects and a decreased range of motion. Early diagnosis and surgical management with resection of the SCJ is critical to ensure successful outcomes for patients. Eradication of the infection is an important principle and is accomplished by a combination of surgical intervention and organism-specific long-term intravenous antibiotics. Multiple studies have demonstrated different surgical techniques.
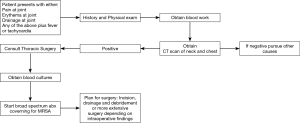
Guidelines for treating these infections are lacking and highlight the importance of developing a structured diagnostic and treatment algorithms that can be used by all physicians. The algorithm developed from our study provides physician and surgeons systematic guidelines that they can follow when diagnosing and treating these types of infections (Figure 3).
An algorithm that can be used when dealing with a suspected SCJ infection includes: (I) performing a thorough history and physical exam along with blood analysis (CBC and blood cultures), and CT scan of the neck and chest with contrast. If these studies demonstrate a possible infection, then the physician should proceed to obtain a thoracic surgery consult. Debridement of the joint is key to early diagnosis for cultures and early treatment. The sooner the patient can get to the operating room for debridement, the sooner the infection can be adequately treated to prevent a worsening infection. (II) Early start of broad-spectrum antibiotics that provide coverage against MRSA infections and early surgical intervention, including joint resection and debridement, is the ideal treatment. (III) Surgical management should include incision, drainage and joint resection. The joint should be resected in all cases. The extent of debridement depends on the surgeon’s evaluation in the operating room. Debridement depends on the extent of erythema and purulent drainage. The infection based on our study is in the joint and patients improve best with the resection of the joint. The need for a muscle flap depends on the size of the deformity and the ability of wound to close by secondary intention. Large deformities that may leave the great vessels and/or pleural space exposed for example would be an indication for a plastic surgery consult for evaluation of the deformity for coverage. A planned return to the operating room for further debridement is always an option, if the wound bed is not completely cleared of infection.
The limitations of this study are that the study is a retrospective chart review and there is a limited amount of data that can be gathered from the chart. Another limitation, is that the study is limited by the infection. SCJ infections are rare and there are not many patients with this infection.
Acknowledgements
None.
Footnote
Conflicts of Interest: This manuscript was presented at the General Thoracic Surgical Club, March 2014.
Ethical Statement: The Institutional Review Board was Loma Linda University. The study was approved by institutional ethics committee board of Loma Linda University (Study #5120311). The Institutional Review Board was the Veterans Affair 01032.
References
- Yood RA, Goldenberg DL. Sternoclavicular joint arthritis. Arthritis Rheum 1980;23:232-9. [Crossref] [PubMed]
- Nusselt T, Klinger HM, Freche S, et al. Surgical management of sternoclavicular septic arthritis. Arch Orthop Trauma Surg 2011;131:319-23. [Crossref] [PubMed]
- Ross JJ, Shamsuddin H. Sternoclavicular septic arthritis: review of 180 cases. Medicine (Baltimore) 2004;83:139-48. [Crossref] [PubMed]
- Burkhart HM, Deschamps C, Allen MS, et al. Surgical management of sternoclavicular joint infections. J Thorac Cardiovasc Surg 2003;125:945-9. [Crossref] [PubMed]
- Song HK, Guy TS, Kaiser LR, et al. Current presentation and optimal surgical management of sternoclavicular joint infections. Ann Thorac Surg 2002;73:427-31. [Crossref] [PubMed]
- Zanelli G, Sansoni S, Migliorini L, et al. Sternoclavicular joint infection in an adult without predisposing risk factors. Infez Med 2003;11:105-7. [PubMed]
- Gillis S, Friedman B, Caraco Y, et al. Septic arthritis of the sternoclavicular joint in healthy adults. J Intern Med 1990;228:275-8. [Crossref] [PubMed]
- Crisostomo RA, Laskowski ER, Bond JR, et al. Septic sternoclavicular joint: a case report. Arch Phys Med Rehabil 2008;89:884-6. [Crossref] [PubMed]
- Carlos GN, Kesler KA, Coleman JJ, et al. Aggressive surgical management of sternoclavicular joint infections. J Thorac Cardiovasc Surg 1997;113:242-7. [Crossref] [PubMed]
- Brook I. Microbiology and management of joint and bone infections due to anaerobic bacteria. J Orthop Sci 2008;13:160-9. [Crossref] [PubMed]
- Puri V, Meyers BF, Kreisel D, et al. Sternoclavicular joint infection: a comparison of two surgical approaches. Ann Thorac Surg 2011;91:257-61. [Crossref] [PubMed]