Minimally invasive aortic valve replacement in high risk patient groups
Introduction
Aortic valve replacement (AVR) via median sternotomy remains the standard treatment for aortic valvular disease. However, the development of minimally invasive approaches in general surgery has driven the adoption of lesser invasive techniques within the field of cardiac surgery. Historically, Cosgrove and Cohn were the first clinicians to pioneer smaller incisions for both mitral and aortic procedures (1). Despite longer cardiopulmonary bypass (CPB) times compared to conventional surgery (1-12), preservation of the sternal integrity and minimisation of dissection had been advocated to improve the cosmetic result, reduce bleeding, provide better respiratory function, yield shorter hospital stays and therefore lower costs and improved patient satisfaction. A large meta-analysis by Phan et al. demonstrated that minimally invasive AVR (mini-AVR) is also associated with a reduced incidence of renal failure and has comparable mortality and morbidity to conventional surgery (13). The above advantages could prove significant in the “high risk” cohorts such as elderly patients, re-operative surgery, poor respiratory status, pulmonary hypertension, renal dysfunction or poor left ventricular (LV) function (14,15). Hence, the current review focuses on the outcomes following mini-AVR in the above high risk groups. Although the definition of minimally invasive approach to the aortic valve remains a matter of debate, the present review discusses the open approaches, performed via a small incision (e.g., not a full sternotomy), therefore we do not refer to the trans-catheter aortic valve implantation (TAVI) (16). We included all the studies where the aortic valve was approached via a minimally invasive approach including: the various types of partial sternotomy, mini-thoracotomy or port-access approach.
Methods
A thorough literature search was conducted in PubMed and Embase databases, using the following search string: “(minimally invasive OR mini OR mini OR minimal access OR partial sternotomy OR hemi-sternotomy OR anterior thoracotomy OR parasternal OR transverse sternotomy) AND (aortic valve) AND (replacement OR surgery OR insertion) AND (high risk OR elderly OR old age OR elderly OR left ventricular failure OR left ventricular dysfunction OR renal failure OR chronic kidney disease OR renal dysfunction OR chronic lung disease OR chronic obstructive pulmonary disease OR re-do OR reoperative OR resternotomy OR reintervention OR pulmonary hypertension)”. Further articles were identified by cross reference check from the articles identified by our search.
Mini-AVR in elderly patients
The increase in the life expectancy has resulted in more aortic valve surgery being performed in the elderly population (17). With an increasing prevalence with age, calcific aortic stenosis (AS) is the main valvular disease in the octogenarians with a predicted requirement for approximately 3,500 AVRs per year in England (17,18). Certainly, conventional AVR (CAVR), performed via full midline sternotomy, remains the definitive treatment and yields excellent results (19). However, elderly patients are less able to cope with the stress of surgery due to a reduced vital organ reserve and various associated comorbidities (18). Several retrospective studies, reported outcomes of mini-AVR versus CAVR in the elderly population (Table 1).

Full table
Grossi et al. (20) compared the outcomes of 166 patients (mean age of 77.5 years) undergoing CAVR, with 56 patients (mean age 76 years) undergoing mini-AVR port access technique. There was no difference in hospital mortality. However, the mini-AVR group had a lower incidence of sepsis and wound complications, decreased fresh frozen plasma transfusion requirement and shorter length of hospital stay.
A prospective study by Sharony et al. (21) matched 189 patients undergoing mini-AVR and 189 patients undergoing CAVR via sternotomy. All patients were aged over 65, with 28% being octogenarians. The two cohorts had similar hospital mortalities and similar rates of: re-intervention, stroke, wound infection, gastro-intestinal complications, new renal failure and respiratory failure. The CPB times were similar but the mini-AVR group had a shorter hospital stay and more patients were discharged to their own home.
The same group (22) reported results of two propensity-matched cohorts, 233 patients each, aged >80 years, undergoing either mini-AVR or CAVR. Hospital mortality and perioperative morbidity was similar in both groups. However, a greater proportion of mini-AVR patients had a reduced length of hospital stay and were discharged home rather than transferred to a rehabilitation facility. In the multivariate analyses, the presence of severe atheromatous aortic diseases and need for urgent operation increased the risk of hospital mortality.
ElBardissi et al. (23) reported outcomes of 249 consecutive mini-AVRs in octogenarians patients that were at a prohibitive high risk (median EuroSCORE of 11% and STS score 10.5%) and were considered candidates for TAVI. Interestingly, the high cardiac surgery risk scores were not predictive of operative mortality which proved to be only 3%. Also, the perioperative morbidity was low: stroke 4%, renal failure requiring dialysis 1%, cardiac arrest 1%, sepsis 1% and pulmonary embolism 1%. The long-term survival of up to 10 years did not differ from a low risk, age and gender matched population.
A retrospective review of consecutive heart operations in patients aged 75 or above, conducted by Lamelas et al. (24), identified 58 patients that underwent mini-AVR performed by mini-thoracotomy and compared them with a cohort of 43 patients that underwent CAVR. The composite of mortality and morbidity was significantly lower in the minimally invasive group. This was due to a lower incidence of renal failure, reduced intubation time, less wound infection and fewer deaths. Furthermore, the intensive care unit (ICU) length of stay and total length of hospital stay were lower in the mini-AVR group.
In a small series of 58 isolated mini-AVR performed via a mini sternotomy in patients with median age of 76, Alassar et al. (25) reported no operative mortality and no late mortality at 6 months. There was one reoperation for bleeding, no pacemaker insertion and no wound infections. The CPB times were acceptable and the ICU mean stay was approximately 2 days and the hospital mean stay was 6 days. Similarly, a larger retrospective study by Krishna et al. (26) on 255 consecutive mini-AVRs, done via mini-right thoracotomy in octogenarians, reported acceptable morbidity and mortality rates.
Santarpino et al. (27) allocated 66 patients undergoing sutureless mini-AVR to two age groups: age ≥80 years (25 patients) and age ≤80 (41 patients). The outcomes in terms of in-hospital mortality, stroke, pacemaker implantation, survival (mean follow-up of approximately 14 months) were not different. A health and wellbeing survey questionnaire was conducted with no differences reported between the groups.
Gilmanov et al. (17) compared two propensity matched groups of isolated mini-AVRs performed via a right anterior thoracotomy (100 patients) versus a full sternotomy (100 patients). Patients were aged 80 years or above. The main findings of the study were reduced stroke incidence, earlier extubation time and shorter hospital stay, favouring the minimally invasive group. Both the in-hospital mortality and long-term survival at 5 years were similar. The rate of postoperative atrial fibrillation (AF) and permanent pacemaker (PPM) insertion rates did not differ.
Moscarelli et al. (28) conducted a systematic review of non-randomised studies of 340 elderly patients (mean population age above 75) who received mini-AVR versus CAVR in 343 patients. They found comparable mortality to full sternotomy, significantly reduced postoperative length of stay, no significant difference in CPB and aortic cross-clamp (AoX) times.
Re-do minimally invasive aortic valve surgery
Several studies investigated the potential benefits of minimally invasive surgery in re-operative aortic valve surgery. Theoretically, a smaller incision would reduce the dissection area resulting in less bleeding from adhesion lysis and less risk of damaging patent coronary grafts. Furthermore, preservation of the sternal integrity in such high-risk patients improves the respiratory function and patients have shown to have shorter hospital stay (29-31) (Table 2).

Full table
In 2000, Byrne et al. (32) reported 34 re-do AVR via partial sternotomy. Sixty-two percent of the patients had previous coronary artery bypass grafting (CABG) while 41% had previous valve surgery. There were no intraoperative deaths and no conversions to full sternotomy. The early mortality was 5.9% due to arrhythmia in one case and a large stroke in the other case. There were no reoperations for bleeding and the median lengths of stays in ICU and hospital were 1 and 7 days respectively.
Sharony et al. (33) reported the outcomes of 161 patients undergoing re-do minimally invasive valve operations via mini-thoracotomy (61 patients had aortic) versus 227 patients having CAVR via median sternotomy (177 patients). The authors found a significantly lower early mortality, no wound infection, less need for transfusion and shorter hospital stays in the minimally invasive group. However, the incidence of congestive heart failure, renal disease and poor LV function was significantly higher in the sternotomy group. Furthermore, the multivariate analysis showed that renal disease and poor ejection fraction were associated with increased mortality. The 5-year morality was comparable between the two groups. No subgroup analysis for the aortic group was performed.
In a smaller series by Bakir et al. (34) of 19 re-do AVR cases done via upper partial sternotomy (63.2% patients with previous coronary CABG) there were no early deaths, however, 4 patients (21%) required return to theatre for bleeding including one from an injury to a previous patent vein graft. The mean follow-up time was 23.6 months and there were two late deaths one of unknown cause and one non-cardiac.
Tabata et al. (35) reported 146 re-do mini AVRs in an elderly population. The majority (93 patients) had previous CABG. The operative mortality was 4.1%, reoperation rates for bleeding was 0.7% and most of the patients required transfusion (83.6%). In this series, there were no CABG graft injuries and the 5-year actuarial survival was 85%. A small series of ten patients with previous CABG undergoing mini-AVR was also reported by Dell’Amore et al. (36). There was no in hospital mortality and no injury to the bypass grafts.
Gaeta et al. (37) reported outcomes following re-operative mini-AVR on 16 patients with previous patent left internal mammary artery (LIMA) grafts. On this small series there were no early deaths but four late deaths were reported, out of which two were due to cardiac causes. No patient required conversion to full sternotomy or reopening for bleeding and there were no injuries to the LIMA grafts.
Mikus et al. (38) in a series of 90 patients who underwent reoperative aortic valve surgery reported comparative results between a minimally invasive approach cohort (38 patients) and a conventional sternotomy cohort (n=52). There were no significant differences between the groups in terms of the profile risk [e.g., EuroSCORE, LV function or body mass index (BMI)]. There was one death in the minimally invasive group. There were no differences in CPB or AoX times between the two groups however the partial sternotomy group had significantly lower ventilation times.
Kaneko et al. (39) reported outcomes following 105 octogenarians that underwent redo isolated AVR. Fifty-one patients underwent mini-AVR while 54 patients had CAVR. Both cohorts had similar risk factors. There was no difference in terms of operative mortality or other postoperative outcomes between the two approaches, however, the survival analysis at 1 and 5 years favoured mini-AVR.
Gosev et al. (40) compared the postoperative outcomes of 34 patients undergoing mini-AVR with 67 patients undergoing CAVR. Both groups did not differ in terms of demographics or preoperative risk profiles. The authors reported shorter operative times, ventilation times, ICU stays and hospital length of stays favouring the mini-AVR group. There was one early death in the CAVR group. Mid-term survival at 1 and 5 years favoured mini-AVR.
In a meta-analysis of seven observational studies of reoperative mini-AVR, Phan et al. (41) found similar in-hospital mortality and stroke rates. The rates of PPM implantation, renal failure and re-operation for bleeding were again similar. There was no difference in hospital stays between the two approaches.
Outcomes of mini AVR in patients with high cardiac risk scores
In the current section we will discuss the studies that reported outcomes of mini-AVR in populations considered high risk according to the various risk scoring systems in cardiac surgery (Table 3).
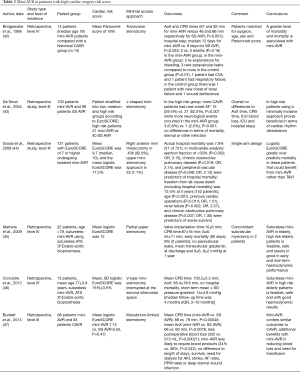
Full table
Bridgewater et al. (42) reported outcomes of a high risk cohort (median age 78 and Parsonnet score of 18%) who underwent mini-AVR via a transverse sternotomy approach compared to a Parsonnet score, age and sex matched retrospective cohort. The authors found a significantly higher mortality, incidence of re-exploration, paravalvular leaks and re-exploration in mini-AVR group. Furthermore, mini-AVR had longer postoperative stays and higher incidence of morbidity.
In 2004, De Smet et al. (43) analysed the outcomes of 100 patients undergoing mini-AVR via J-sternotomy compared to a retrospective series of CAVR performed in 91 patients operated before introduction of mini-AVR in that institution. Both cohorts had similar preoperative characteristics and they were further stratified by EuroSCORE in low, medium and high risk. In the high risk group (EuroSCORE >6) there was a higher incidence of AF in patients undergoing sternotomy in contrast to mini-AVR patients who experienced more neurologic events. However, when only the severe brain injuries were included in the analysis no difference was noted. Similarly, a greater incidence of AF occurred in the medium risk sternotomy group. In the low risk mini-AVR patients, there was a higher incidence of AF. Overall, the mortality and lengths of stay were similar between conventional sternotomy versus mini-sternotomy.
Grossi et al. (44) reported outcomes of isolated AVR in a high risk cohort of 731 patients with mean EuroSCORE of 9.7. Mini-AVR was performed in 64.2% (469 patients). No comparative analysis was performed between the operative approaches. The actual hospital mortality was 7.8% suggesting that the EuroSCORE over predicted the mortality. In the multivariate analysis; poor ejection fraction, chronic obstructive pulmonary disease (COPD) and peripheral vascular disease significantly affected hospital mortality. The 5-year freedom from all-cause mortality was 72.4% at 5 years. Age, reoperation, renal and chronic lung disease were predictors of worse survival.
Martens et al. (45) reported outcomes of mini-AVR via partial sternotomy, using the ATS 3f Enable sutureless bioprosthesis, in 22 elderly patients. Mean age of 75 years and mean logistic EuroSCORE of 13. The mean reimplantation time was 10±6 min, CPB time was 87 min and the mean cross clamp time was 55 min. The early mortality (<90 days) was 9% (2 deaths). There were no paravalvular leaks and the implanted valves had low gradients both on discharge and at 12 months.
Later, in 2013, Concistre et al. (46) reported outcomes in an elderly population (mean age of 77) undergoing sutureless aortic valve implantation (3f Enable bioprosthesis) via V-type mini-sternotomy. The mean EuroSCORE of the cohort was 15%. The mean CPB and aortic cross clamp times were 100.2 and 66.4 min respectively. One patient had trivial paravalvular leak and there were no early deaths or at follow-up (median follow-up time was 4 months, interquartile range, 2–10 months). The mean pressure gradients were remained low on follow-up. Burdett et al. (47) compared two matched cohorts for perioperative profile and risk score (mean EuroSCORE of 7). One group (98 patients) underwent manubrium limited sternotomy and the other conventional sternotomy (93 patients). The mini-AVR cohort had longer CPB times and aortic cross clamp times (10 and 6 min respectively) but significantly less postoperative blood loss and transfusion requirements. The postoperative morbidity, length of stays, rate of paravalvular leaks and in hospital mortality were similar.
Mini AVR in patients with chronic lung disease or pulmonary hypertension
As discussed earlier, maintenance of the sternal integrity could prove beneficial in patients with reduced respiratory reserve. Calderon et al. (48) in a prospective randomized trial of mini-AVR versus CAVR measured the postoperative spirometry parameters in 78 patients. They found no significant changes between the two groups.
Stolinski et al. (49) measured the pulmonary function tests at 1 week, 1 month and 3 months of two elderly cohorts (mean age >75 years): mini-AVR (65 patients) versus CAVR (82 patients). The two cohorts had similar perioperative characteristics. At 1 week and 1 month the pulmonary function was better in the mini-AVR but there was no significant difference between the two groups at 3 months. The duration of postoperative mechanical ventilation was lower in the mini-AVR group but the incidence of pulmonary complications was similar.
A study by Li et al. (50) measured the extravascular water index and pulmonary vascular permeability index in 90 patients that received either a conventional sternotomy, mini-AVR via right anterior thoracotomy or via upper sternotomy. The minimally invasive groups had a faster recovery of the above parameters.
Only a few studies evaluating the effect of mini-AVR in patients with poor lung function pre-operatively exist. Albacker et al. (51) in a propensity matched study of 223 patient pairs: mini-AVR via J-sternotomy versus AVR via full sternotomy found a shorter ICU and hospital length of stay for mini-AVR patients as the forced expiratory volume in 1 second (FEV1) decreased. There was a trend towards higher survival of mini-AVR patients (93% versus 89% at 1 year, P=0.07) however there was no difference in late survival.
A retrospective analysis of 165 patients with COPD (82% had moderate COPD, e.g., FEV1 between 50% and 80%) by Santana et al. (52) found no difference in hospital mortality between the mini-AVR patients (n=100) and the conventional sternotomy patients (n=65). However, the composite of post-operative complications was significantly reduced in mini-AVR groups. Furthermore, the ICU lengths of stay and hospital length of stay were shorter in minimally invasive group.
Gosain et al. (53) reported outcomes after mini-AVR in 569 patients with pulmonary hypertension. The mean pulmonary artery pressure of the group was 33 mmHg. The overall early mortality was 3.5% and the stroke rates were 1.4%. Patients with severe pulmonary hypertension had a significantly longer ICU stay and trend towards longer ventilation times.
Mini AVR in patients with renal dysfunction
Pre-operative renal dysfunction is an independent risk factor in operative mortality and late survival in patients undergoing heart surgery (54). The renoprotective effect of minimally invasive surgery has already been proven in mitral valve surgery (55,56).
We found only two studies that evaluated the effect mini-AVR in patients with pre-existing renal dysfunction. Valdez et al. (57) retrospectively reviewed a cohort of 688 patients with chronic kidney disease stages 2–5. In their study, 236 patients received a mini-AVR and 87 received a CAVR. There were no differences in operative mortality between the two groups. The mini-AVR group had a lower incidence of acute on chronic kidney injury despite longer CPB and AoX times. However, there was no difference in the peak postoperative creatinine measurements between the two groups or the need for dialysis. The authors used the RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) classification to define acute kidney injury (AKI). This classification also takes into account urine output and the estimated glomerular filtration rate. Furthermore, the mini-AVR patients had fewer composite complications, shorter ICU and hospital lengths of stay. In the multivariate analysis minimally invasive surgery was associated with 60% reduction in the risk of development of AKI. Similar results were found by a large meta-analysis of non-randomized controlled trials where renal failure occurred less in the mini-AVR group despite longer CPB and AoX times (13).
Haldenwang et al. (58), in a small retrospective study compared 77 patients that received a mini-AVR to 56 patients that received a TAVI. The mini AVR patients had a lower risk of developing AKI compared to TAVI.
Patients with poor LV function or severe heart failure
The study by Tabata et al. (59) is the only one to date that compares the effect of mini-AVR versus CAVR in patients with pre-operative LV dysfunction. The authors propensity matched two cohorts of 41 patients each (mini-AVR or CAVR). There was no significant difference in operative mortality, post-operative complications, blood transfusion requirement or length of hospital stay and CPB and AoX times.
A study conducted by Mihaljevic et al. (60) compared two propensity matched cohorts of patients with severe heart failure (New York Heart Association III and IV): minimally invasive valve surgery versus conventional sternotomy valve surgery. The mean ejection fraction did not differ between the groups and was classed as moderate. The comparisons were adjusted not only to the patient characteristics but also to the individual surgeon. Without adjusting for the operator, the CPB, aortic cross clamp times and ICU length stays were shorter for the minimally invasive group. The hospital mortality, long-term survival were similar. However, when adjusting for the surgeon there were no differences in the outcomes between the two groups.
Mini AVR in obese patients
Several studies found no adverse outcomes of performing conventional cardiac surgery in obese patients. However, this group of patients is at increased risk of deep sternal wound infection, therefore a minimally invasive approach could prove advantageous (61,62). In contrast, adequate exposure using a minimally invasive approach can prove to be a challenge. Two studies to our knowledge evaluated the effect of mini-AVR in this high-risk group (Table 4). Santana et al. (62) compared the outcomes of 31 patients who had mini-AVR via a mini-thoracotomy with a matched group 43 patients had CAVR. The composite of postoperative complications occurred less frequently in the minimally invasive group. This was driven by a lower incidence of renal failure, shorter ventilation times, lower reintubation rates, lower incidence of deep sternal wound infection and reduced in-hospital mortality. A recent study by Acharya et al. (63) on 90 patients who underwent mini-AVR compared using univariate regression analysis on the effect on postoperative outcomes of a BMI <25 (in 36 patients) with the effect of BMI ≥25 (54 patients). The high BMI cohort had increased incidence of hospital acquired pneumonia and new onset of AF. However, there was no difference in ICU length of stay, hospital length of stay, wound complications rates, inotrope requirements or renal dysfunction. Furthermore, a correlation between increasing BMI and reduced ventilation or post-operative blood loss was found.

Full table
Mini-AVR in patients requiring concomitant procedures
Another high-risk group is that of patients requiring additional, complex procedures associated to minimally aortic valve surgery.
Totaro et al. (64) reported the outcomes of 1,126 procedures performed via upper mini-sternotomy. The authors compared the outcomes of isolated mini-AVR (61%) with re-do mini-AVR (7%) or other complex cardiac surgery (32%) including AVR combined with aortic surgery procedures or CABG. The complex cardiac surgery group had a higher operative mortality, longer ventilation times and longer ICU status however the surgical revision rates were similar in all three groups.
Kaneko et al. (65) reported outcomes of mini-AVR via an upper hemi-sternotomy in 119 patients who required a concomitant aortic procedure. The majority of the patients (59.6%) had supra-coronary ascending aorta replacement. The authors reported an operative mortality of 2.8% and a postoperative survival at 1 and 5 years of 96.2% and 92.4% respectively. There were 4 (3.7%) reoperations for bleeding. Other complications included postoperative renal failure in two cases and myocardial infarction in two other cases. The mean length of stay was approximately a week.
Elmahdy et al. (66) reported a case series of six patients that had triple valve surgery via a right anterior mini-thoracotomy. All patients had aortic valve surgery, mitral valve repair and tricuspid valve repair. The authors reported two early deaths and two cases of postoperative AF.
Sutureless mini-AVR in high risk patients
Rapid deployment valves (RDV) or sutureless valves had been developed with excellent perioperative outcomes and are increasingly used (67-70). Currently, RDV-AVR can be performed using either the self-expanding Perceval S (LivaNova Group, Milan, Italy) or the rapid-deployment Intuity Elite (Edward Lifesciences, Irvine, USA) (71). Some authors showed that the AoX and CPB times are independent predictors of mortality and morbidity after cardiac surgery (72). Hence, the reduced time taken to deploy these valves could potentially translate into better outcomes, especially in the high-risk patients. Phan et al. in a meta-analysis of observational studies found RDV-AVR to be safe and associated with shorter AoX and CPB duration and with comparable complication rates to the conventional approach in the short-term (70). Furthermore, the ease of placement of these valves makes them suitable to be inserted using a minimally invasive approach (70,71). Therefore, minimally invasive RDV-AVR could prove to be a significant competitor of TAVI, particularly in the high risk patients. In a recent multicenter propensity matched study comparing outcomes of 214 patients matched to either RDV-AVR or TAVI, there was no difference in 30-day or 1-year mortality, stroke, bleeding or myocardial infarction (73). However, the RDV-AVR patients had a higher procedural success rate and less incidence of paravalvular leaks at the cost of higher incidence of pacemaker insertion compared to the TAVI cohort. Conversely, the TAVI cohort had a significantly shorter ICU stay and hospitalization duration and significantly lower peak and mean aortic gradients. The main advantage of RDV-AVR is the resection of the native valve and the annular decalcification that could translate into better haemodynamic profiles and possibly better long-term outcomes, which would be pertinent in younger patients (71). Similarly to TAVI literature, there is a paucity of long-term follow-up studies of RDV-AVR. Englberger et al. (68) reported excellent haemodynamic profiles, very low re-operation rates and no structural valve deterioration at 5 years, in a cohort of 141 patients undergoing RDV-AVR.
Patients with small aortic root are prone to patient prosthetic mismatch which could result in poorer outcomes (74). In high-risk patients, adding aortic root enlargement with prolongation of surgery may lead to detrimental effects. Hence, the use of RDV may prove to be a better strategy (75). Other groups that can benefit from RDV-AVR are the patients with severe aortic root calcification (76) or re-do AVR (77) where inserting stitches for conventional valves may prove to be very difficult or sometimes virtually impossible. Certainly, well designed randomized trials comparing TAVI with RDV-mini-AVR with long-term follow-up are needed to find out the best alternative in high risk cohorts.
Limitations of the current evidence
The evidence presented in the current review suggests that mini-AVR in the various high risk categories can be performed with comparable survival to conventional techniques but with several additional benefits. However, we have to acknowledge several limitations to the conclusions we draw. Firstly, most of the studies we found were single centre, non-randomized and retrospective. Furthermore, both the patients and medical staff were not blinded to the treatment modality. Efforts were made to balance the groups by propensity matching or cardiac risk score matching in the comparative studies, but this does eliminate patient selection bias entirely. We also found heterogeneity of the available data in terms of the postoperative outcomes. Some studies showed that mini-AVR could reduce various events such as renal failure transfusions etc. in contrast to other studies that did not show this. Very few studies included other valve surgery that lacked sub-analysis for aortic procedures. In this review, we also looked at series of mini-AVR using sutureless aortic valves. In such cases, the improved outcomes could well be attributed to the sutureless valve rather than the minimally invasive approach. Finally, there was heterogeneity in the type of minimally invasive approach used that could influence the outcomes.
Conclusions
All the current evidence on the performance of mini-AVR in high risk patient groups is based on retrospective, observational studies. In all high-risk groups, mini-AVR is performed with comparable morality and mid-term survival to CAVR. In elderly patients, despite longer AoX and CPB times, mini-AVR results in improved ventilatory function and renal function, reduced wound infection, shorter hospitalization and a greater proportion of patient being discharged straight to home. Re-do mini-AVR is a safe procedure with some studies showing a benefit in mid-term survival. Mini-sternotomy in patients with previous CABG can be performed with a low risk of injury to patent grafts. Current cardiac surgery scoring systems tend to overestimate mortality in high risk patients. Despite longer operative times and longer hospitalization, RDV-mini-AVR is a competitive alternative to TAVI in the high-risk patient. In patients with chronic lung disease, pulmonary hypertension or chronic kidney disease, a minimally invasive approach is safe and reduces hospital and ICU length of stays. Obese patients can benefit from a minimally invasive approach in terms of reduced wound complications, improved respiratory function and improved survival according to a study. Mini-AVR concomitant with aortic surgery or valve surgery can be performed with acceptable mortality and morbidity.
Acknowledgments
Funding: The study was supported by the British Heart Foundation and the NIHR Bristol Cardiovascular Biomedical Research Unit (No. RJ6520).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Soltesz EG, Cohn LH. Minimally invasive valve surgery. Cardiol Rev 2007;15:109-15. [Crossref] [PubMed]
- Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-9.e5. [Crossref] [PubMed]
- Ghanta RK, Lapar DJ, Kern JA, et al. Minimally invasive aortic valve replacement provides equivalent outcomes at reduced cost compared with conventional aortic valve replacement: A real-world multi-institutional analysis. J Thorac Cardiovasc Surg 2015;149:1060-5. [Crossref] [PubMed]
- Johnston DR, Atik FA, Rajeswaran J, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. J Thorac Cardiovasc Surg 2012;144:852-8.e3. [Crossref] [PubMed]
- Khoshbin E, Prayaga S, Kinsella J, et al. Mini-sternotomy for aortic valve replacement reduces the length of stay in the cardiac intensive care unit: meta-analysis of randomised controlled trials. BMJ Open 2011;1:e000266. [Crossref] [PubMed]
- Neely RC, Boskovski MT, Gosev I, et al. Minimally invasive aortic valve replacement versus aortic valve replacement through full sternotomy: the Brigham and Women's Hospital experience. Ann Cardiothorac Surg 2015;4:38-48. [PubMed]
- Mächler HE, Bergmann P, Anelli-Monti M, et al. Minimally invasive versus conventional aortic valve operations: A prospective study in 120 patients. Annals of Thoracic Surgery 1999;67:1001-5. [Crossref] [PubMed]
- Bonacchi M, Prifti E, Giunti G, et al. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg 2002;73:460-5; discussion 5-6. [Crossref] [PubMed]
- Mihaljevic T, Cohn LH, Unic D, et al. One Thousand Minimally Invasive Valve Operations: Early and Late Results. Annals of Surgery 2004;240:529-34. [Crossref] [PubMed]
- Cohn LH. Minimally invasive valve surgery. Journal of cardiac surgery 2001;16:260-5. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-Invasive Valve Surgery. Journal of the American College of Cardiology 2010;56:455-62. [Crossref] [PubMed]
- Scarci M, Young C, Fallouh H. Is ministernotomy superior to conventional approach for aortic valve replacement? Interactive CardioVascular and Thoracic Surgery 2009;9:314-7. [Crossref] [PubMed]
- Phan K, Xie A, Di Eusanio M, et al. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg 2014;98:1499-511. [Crossref] [PubMed]
- Schmitto JD, Mohr FW, Cohn LH. Minimally invasive aortic valve replacement: how does this perform in high-risk patients? Current Opinion in Cardiology 2011;26:118-22. [Crossref] [PubMed]
- Attia RQ, Hickey GL, Grant SW, et al. Minimally Invasive Versus Conventional Aortic Valve Replacement: A Propensity-Matched Study From the UK National Data. Innovations (Phila) 2016;11:15-23; discussion 23.
- Glauber M, Miceli A. Minimally invasive aortic valve replacement with sutureless valve is the appropriate treatment option for high-risk patients and the "real alternative" to transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2016;151:610-3. [Crossref] [PubMed]
- Gilmanov D, Farneti PA, Ferrarini M, et al. Full sternotomy versus right anterior minithoracotomy for isolated aortic valve replacement in octogenarians: a propensity-matched study dagger. Interact Cardiovasc Thorac Surg 2015;20:732-41; discussion 41. [Crossref] [PubMed]
- Prêtre R, Turina MI. Cardiac valve surgery in the octogenarian. Heart 2000;83:116-21. [Crossref] [PubMed]
- Stoica SC, Cafferty F, Kitcat J, et al. Octogenarians undergoing cardiac surgery outlive their peers: a case for early referral. Heart 2006;92:503-6. [Crossref] [PubMed]
- Grossi EA, Galloway AC, Ribakove GH, et al. Minimally invasive port access surgery reduces operative morbidity for valve replacement in the elderly. Heart Surg Forum 1999;2:212-5. [PubMed]
- Sharony R. Minimally Invasive Aortic Valve Surgery in the Elderly: A Case-Control Study. Circulation 2003;108 Suppl 1:II43-7. [Crossref] [PubMed]
- Sharony R, Grossi EA, Saunders PC, et al. Propensity score analysis of a six-year experience with minimally invasive isolated aortic valve replacement. J Heart Valve Dis 2004;13:887-93. [PubMed]
- ElBardissi AW, Shekar P, Couper GS, et al. Minimally invasive aortic valve replacement in octogenarian, high-risk, transcatheter aortic valve implantation candidates. J Thorac Cardiovasc Surg 2011;141:328-35. [Crossref] [PubMed]
- Lamelas J, Sarria A, Santana O, et al. Outcomes of minimally invasive valve surgery versus median sternotomy in patients age 75 years or greater. Ann Thorac Surg 2011;91:79-84. [Crossref] [PubMed]
- Alassar Y, Yildirim Y, Pecha S, et al. Minimal access median sternotomy for aortic valve replacement in elderly patients. J Cardiothorac Surg 2013;8:103. [Crossref] [PubMed]
- Krishna RK, Santana O, Mihos CG, et al. Minimally invasive aortic valve replacement in octogenarians performed via a right anterior thoracotomy approach. J Heart Valve Dis 2014;23:671-4. [PubMed]
- Santarpino G, Pfeiffer S, Vogt F, et al. Advanced age per se should not be an exclusion criterion for minimally invasive aortic valve replacement. J Heart Valve Dis 2013;22:455-9. [PubMed]
- Moscarelli M, Emmanuel S, Athanasiou T, et al. The role of minimal access valve surgery in the elderly. A meta-analysis of observational studies. Int J Surg 2016;33 Pt A:164-71.
- Byrne JG, Aranki SF, Couper GS, et al. Reoperative aortic valve replacement: partial upper hemisternotomy versus conventional full sternotomy. J Thorac Cardiovasc Surg 1999;118:991-7. [Crossref] [PubMed]
- Pineda AM, Santana O, Lamas GA, et al. Is a minimally invasive approach for re-operative aortic valve replacement superior to standard full resternotomy? Interact Cardiovasc Thorac Surg 2012;15:248-52. [Crossref] [PubMed]
- Kaneko T, Leacche M, Byrne J, et al. Reoperative minimal access aortic valve replacement. J Thorac Dis 2013;5 Suppl 6:S669-72. [PubMed]
- Byrne JG, Karavas AN, Adams DH, et al. Partial upper re-sternotomy for aortic valve replacement or re-replacement after previous cardiac surgery. Eur J Cardiothorac Surg 2000;18:282-6. [Crossref] [PubMed]
- Sharony R, Grossi EA, Saunders PC, et al. Minimally invasive reoperative isolated valve surgery: early and mid-term results. J Card Surg 2006;21:240-4. [Crossref] [PubMed]
- Bakir I, Casselman FP, De Geest R, et al. Should minimally invasive aortic valve replacement be restricted to primary interventions? Thorac Cardiovasc Surg 2007;55:304-9. [Crossref] [PubMed]
- Tabata M, Khalpey Z, Shekar PS, et al. Reoperative minimal access aortic valve surgery: minimal mediastinal dissection and minimal injury risk. J Thorac Cardiovasc Surg 2008;136:1564-8. [Crossref] [PubMed]
- Dell'Amore A, Del Giglio M, Calvi S, et al. Mini re-sternotomy for aortic valve replacement in patients with patent coronary bypass grafts. Interact Cardiovasc Thorac Surg 2009;9:94-7. [Crossref] [PubMed]
- Gaeta R, Lentini S, Raffa G, et al. Aortic valve replacement by ministernotomy in redo patients with previous left internal mammary artery patent grafts. Ann Thorac Cardiovasc Surg 2010;16:181-6. [PubMed]
- Mikus E, Calvi S, Tripodi A, et al. Upper 'J' Ministernotomy versus Full Sternotomy: An Easier Approach for Aortic Valve Reoperation. Journal of Heart Valve Disease 2013;22:295-300. [PubMed]
- Kaneko T, Loberman D, Gosev I, et al. Reoperative aortic valve replacement in the octogenarians-minimally invasive technique in the era of transcatheter valve replacement. J Thorac Cardiovasc Surg 2014;147:155-62. [Crossref] [PubMed]
- Gosev I, Neely RC, Leacche M, et al. The impact of a minimally invasive approach on reoperative aortic valve replacement. J Heart Valve Dis 2015;24:181-6. [PubMed]
- Phan K, Zhou JJ, Niranjan N, et al. Minimally invasive reoperative aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:15-25. [PubMed]
- Bridgewater B, Steyn RS, Ray S, et al. Minimally invasive aortic valve replacement through a transverse sternotomy: a word of caution. Heart 1998;79:605-7. [Crossref] [PubMed]
- De Smet JM, Rondelet B, Jansens JL, et al. Assessment based on EuroSCORE of ministernotomy for aortic valve replacement. Asian Cardiovasc Thorac Ann 2004;12:53-7. [Crossref] [PubMed]
- Grossi EA, Schwartz CF, Yu PJ, et al. High-risk aortic valve replacement: are the outcomes as bad as predicted? Ann Thorac Surg 2008;85:102-6; discussion 7. [Crossref] [PubMed]
- Martens S, Ploss A, Sirat S, et al. Sutureless aortic valve replacement with the 3f Enable aortic bioprosthesis. Ann Thorac Surg 2009;87:1914-7. [Crossref] [PubMed]
- Concistrè G, Miceli A, Chiaramonti F, et al. Sutureless aortic valve implantation through an upper v-type ministernotomy: an innovative approach in high-risk patients. Innovations (Phila) 2013;8:23-8. [Crossref] [PubMed]
- Burdett CL, Lage IB, Goodwin AT, et al. Manubrium-limited sternotomy decreases blood loss after aortic valve replacement surgery. Interact Cardiovasc Thorac Surg 2014;19:605-10. [Crossref] [PubMed]
- Calderon J, Richebe P, Guibaud JP, et al. Prospective randomized study of early pulmonary evaluation of patients scheduled for aortic valve surgery performed by ministernotomy or total median sternotomy. J Cardiothorac Vasc Anesth 2009;23:795-801. [Crossref] [PubMed]
- Stoliński J, Plicner D, Gawęda B, et al. Function of the Respiratory System in Elderly Patients after Aortic Valve Replacement. J Cardiothorac Vasc Anesth 2016;30:1244-53. [Crossref] [PubMed]
- Li W, Xue Q, Liu K, et al. Effects of MIAVS on Early Postoperative ELWI and Respiratory Mechanics. Medical Science Monitor 2016;22:1085-92. [Crossref] [PubMed]
- Albacker TB, Blackstone EH, Williams SJ, et al. Should less-invasive aortic valve replacement be avoided in patients with pulmonary dysfunction? J Thorac Cardiovasc Surg 2014;147:355-61.e5. [Crossref] [PubMed]
- Santana O, Reyna J, Benjo AM, et al. Outcomes of minimally invasive valve surgery in patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2012;42:648-52. [Crossref] [PubMed]
- Gosain P, Larrauri-Reyes M, Mihos CG, et al. Aortic and/or mitral valve surgery in patients with pulmonary hypertension performed via a minimally invasive approach. Interact Cardiovasc Thorac Surg 2016;22:668-70. [Crossref] [PubMed]
- Howell NJ, Keogh BE, Bonser RS, et al. Mild renal dysfunction predicts in-hospital mortality and post-discharge survival following cardiac surgery. Eur J Cardiothorac Surg 2008;34:390-5; discussion 5. [Crossref] [PubMed]
- Antonic M, Gersak B. Renal function after port access and median sternotomy mitral valve surgery. Heart Surg Forum 2007;10:E401-7. [Crossref] [PubMed]
- McCreath BJ, Swaminathan M, Booth JV, et al. Mitral valve surgery and acute renal injury: port access versus median sternotomy. Ann Thorac Surg 2003;75:812-9. [Crossref] [PubMed]
- Valdez GD, Mihos CG, Santana O, et al. Incidence of postoperative acute kidney injury in patients with chronic kidney disease undergoing minimally invasive valve surgery. J Thorac Cardiovasc Surg 2013;146:1488-93. [Crossref] [PubMed]
- Haldenwang P, Trampisch M, Schlomicher M, et al. Risk factors for acute kidney injury following TA-TAVI or minimally invasive aortic valve replacement: which procedure is less kidney damaging in elderly patients? Thorac Cardiovasc Surg 2014;62:482-8. [Crossref] [PubMed]
- Tabata M, Aranki SF, Fox JA, et al. Minimally invasive aortic valve replacement in left ventricular dysfunction. Asian Cardiovasc Thorac Ann 2007;15:225-8. [Crossref] [PubMed]
- Mihaljevic T, Planinc M, Williams SJ, et al. Less invasive versus conventional heart valve surgery in patients with severe heart failure. J Thorac Cardiovasc Surg 2014;148:161-7.e6. [Crossref] [PubMed]
- Rockx MA, Fox SA, Stitt LW, et al. Is obesity a predictor of mortality, morbidity and readmission after cardiac surgery? Can J Surg 2004;47:34-8. [PubMed]
- Santana O, Reyna J, Grana R, et al. Outcomes of minimally invasive valve surgery versus standard sternotomy in obese patients undergoing isolated valve surgery. Ann Thorac Surg 2011;91:406-10. [Crossref] [PubMed]
- Acharya M, Harling L, Moscarelli M, et al. Influence of body mass index on outcomes after minimal-access aortic valve replacement through a J-shaped partial upper sternotomy. J Cardiothorac Surg 2016;11:74. [Crossref] [PubMed]
- Totaro P, Carlini S, Pozzi M, et al. Minimally invasive approach for complex cardiac surgery procedures. Ann Thorac Surg 2009;88:462-6; discussion 7. [Crossref] [PubMed]
- Kaneko T, Couper GS, Borstlap WA, et al. Minimal-access aortic valve replacement with concomitant aortic procedure: a 9-year experience. Innovations (Phila) 2012;7:368-71. [Crossref] [PubMed]
- Elmahdy HM, Nascimento FO, Santana O, et al. Outcomes of minimally invasive triple valve surgery performed via a right anterior thoracotomy approach. J Heart Valve Dis 2013;22:735-9. [PubMed]
- Breitenbach I, Wimmer-Greinecker G, Bockeria LA, et al. Sutureless aortic valve replacement with the Trilogy Aortic Valve System: multicenter experience. J Thorac Cardiovasc Surg 2010;140:878-84, 84.e1.
- Englberger L, Carrel TP, Doss M, et al. Clinical performance of a sutureless aortic bioprosthesis: five-year results of the 3f Enable long-term follow-up study. J Thorac Cardiovasc Surg 2014;148:1681-7. [Crossref] [PubMed]
- Sadowski J, Kapelak B, Pfitzner R, et al. Sutureless aortic valve bioprothesis '3F/ATS Enable'--4.5 years of a single-centre experience. Kardiol Pol 2009;67:956-63. [PubMed]
- Phan K, Tsai YC, Niranjan N, et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:100-11. [PubMed]
- Mazine A, Bonneau C, Karangelis D, et al. Sutureless aortic valves: who is the right patient? Curr Opin Cardiol 2017;32:130-6. [PubMed]
- Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814-22. [Crossref] [PubMed]
- D'Onofrio A, Salizzoni S, Rubino AS, et al. The rise of new technologies for aortic valve stenosis: A comparison of sutureless and transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2016;152:99-109.e2. [Crossref] [PubMed]
- Pibarot P, Weissman NJ, Stewart WJ, et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. J Am Coll Cardiol 2014;64:1323-34. [Crossref] [PubMed]
- Villa E, Messina A, Laborde F, et al. Challenge for perceval: aortic valve replacement with small sutureless valves--a multicenter study. Ann Thorac Surg 2015;99:1248-54. [Crossref] [PubMed]
- Capestro F, Massaccesi S, Matteucci ML, et al. Sutureless aortic valve prosthesis in a calcified homograft. J Thorac Cardiovasc Surg 2015;150:e29-30. [Crossref] [PubMed]
- Villa E, Messina A, Cirillo M, et al. Perceval sutureless valve in freestyle root: new surgical valve-in-valve therapy. Ann Thorac Surg 2013;96:e155-7. [Crossref] [PubMed]


