Pediatric airway surgery
The pediatric airway—general implications
The pediatric airway has several distinct features compared to the adult airway. First, dimensions are much smaller. This is particularly important with regards to the subglottis. The inner-diameter of the subglottis of a newborn usually measures between 3 and 4 mm (1). A pathological process in this area, leading to a circular lumen reduction of only one millimeter, can result in a life-threatening near-occlusion.
A second feature of the pediatric airway is that the cartilaginous parts are still malleable and cannot provide the same stability as an adult airway skeleton. This can become a problem after airway reconstructions and usually results in the need of prolonged postoperative stentings to support a laryngotracheal repair. The lack of stability of the trachea in a newborn (compared to an adult) also makes it more prone to develop tracheomalacia. Vessel malformations (vascular rings) can apply pressure to the airway, causing dynamic collapse and making surgical correction often inevitable (2).
On the other hand, the flexibility of the pediatric trachea facilitates long-segmental resections. In children, up to 50 percent of the airway length can be resected without extensive tension on the anastomosis (3).
With regards to the functional aspects of the pediatric larynx, the anatomical relationship between the glottis and the hyoid is also a unique feature. The thyrohyoid membrane is shorter than in adults and the thyroid notch usually projects behind the hyoid. This high location of the larynx raises the tip of the epiglottis behind the uvula, thus, permitting simultaneous breathing and swallowing (4). Consequently, swallowing problems are rare in children and the pediatric larynx can handle even profound changes in its configuration. In fact, extensive laryngotracheal resections and reconstructions are possible without risking severe postoperative swallowing problems.
Diseases of the pediatric airway
Laryngotracheal stenosis
Airway injury secondary to traumatic intubation remains the most common cause of laryngotracheal stenosis, accounting for about 90% of all pediatric acquired airway stenosis (5). The majority of intubation-related injuries are due to the use of an endotracheal tube being too big for the neonatal airway. Although there are no clear guidelines it is generally agreed that the smallest tube providing adequate ventilation should be used. The slightest resistance met during intubation should lead to downsizing to a smaller tube size. Tubes armed with a stylet have to be used with caution, since they can cause significant injury. In most centers cuffed tubes are only used when intubation is required for a short period of time. Cuff-less tubes are considered less traumatic and are preferred for long-time intubation. The right time for tracheostomy in infants and children requiring long-term respiratory support is still a matter of discussion. Endotracheal tubes are usually better tolerated in children than in adults, especially if nasotracheal tubes are used. Median time periods of up to three months before trachestomy have been reported in the literature with reasonable outcome (6).
There are two areas of the pediatric larynx that are prone to pressure-related injury: (I) the posterior commissure of the larynx; and (II) the subglottis. Injury to either of the two areas is associated with a unique pathology. Damage to the posterior commissure leads to scarification posteriorly and fixation of the cricoarytenoid joints (posterior glottic stenosis). Pressure-related necrosis of the subglottic mucosa usually leads to circumferential strictures at the level of the cricoid.
The extent of laryngotracheal stenosis is classified using the modified Myer-Cotton grading system. This grading system incorporates the severity of stenosis, its extension into the glottis as well as additional comorbidities. Comorbidities include severe congenital cardiovascular abnormalities, neurological impairment or syndromic anomalies. Successful decannulation rates after repair decrease from 97% in the isolated subglottic stenosis group without comorbidities to only 64% in children with a glotto-subglottic stenosis and significant comorbidities (Table 1).
Full table
Post-tracheostomy-stenosis/malacia
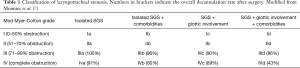
In children the appropriate height of a tracheostomy is essential. If the indication for a tracheostomy is a laryngotracheal stenosis, it should ideally be placed at the level of the first tracheal ring. This enables the surgeon to include the tracheostomy tract in the resection during a later repair. However, in children with longer neck, the tracheostomy can also be placed low in the neck. For all other indications (non-laryngotracheal stenosis) a high tracheostomy should generally be avoided (8). Long-term complications after tracheostomy in children are comparable to adults. An indwelling tracheostomy cannula can either lead to a local destabilization of the airway (tracheomalacia) or a stenosis by scarred retraction (Figure 1). Unlike adults, where symptoms develop gradually within weeks/months after a successful decannulation, children often cannot tolerate removal of the tracheostomy cannula when there is an underlying problem. In these cases a thorough examination of the diseased segment as well as of the laryngeal function is pivotal for a successful repair.
Localized tracheomalacia—vascular rings
Extrinsic compression of the airway by cardiovascular anomalies usually leads to the development of localized tracheomalacia. By definition, vascular rings can either be incomplete (56%) or complete (38%) (9). The most common cause of localized tracheomalacia in children is an aberrant brachiocephalic artery leading to compression of the right anterolateral aspect of the trachea (incomplete ring). The generally accepted treatment for this anomaly is aortopexy (10). Complete vascular rings (vascular slings, double aortic arch) or other complex abnormalities are challenging malformations often requiring complicated cardiovascular reconstructions with concomitant airway repair (11-13). Endotracheal stents for tracheomalacia are associated with minimal immediate procedural risk and could therefore be considered as an alternative in highly selected cases. Furthermore, stenting poses a second-line treatment after failed surgical correction (14). However, long-term success is limited by formation of granulation tissue, recurrent infections and secondary cicatricial stenosis (15).
An important differential diagnosis of localized tracheomalacia/vascular rings is diffuse congenital tracheomalacia in preterm infants, which is caused by an immaturity of cartilage. In most cases the airway skeleton stabilizes with growing and supportive measures as humidifying air are usually sufficient to overcome this condition.
Esophageal atresia and tracheo-esophageal fistula
According the Gross classification, five types of esophageal atresia can be differentiated (16). In 90% of all cases, esophageal atresia is associated with tracheo-esophageal fistula (17). The most common type consists of a blind-ended proximal esophageal pouch with the distal esophagus originating from a distal tracheal fistula. Symptoms include aspiration pneumonia, excessive coughing of mucus with worsening during feeding. Diagnosis is usually established by bronchoscopy. Additionally, fluoroscopy can be used to visualize the fistula. Tracheo-esophageal fistula can be associated with localized tracheomalacia. The required type of repair depends on the size of the gap between the two esophageal ends. In the vast majority of cases a primary esophagoesophagostomy can be performed (18). Long-gap defects can be treated by replacing the esophagus by a gastric conduit (19).
Laryngeal/laryngotracheal clefts
Laryngeal and laryngotracheal clefts are rare congenital defects with an insufficient division of the respiratory tract and the upper digestive tract (20). They can be classified depending on the extent of the affected airway, reaching from rather simple interarytenoid clefts (type I) to extensive laryngotracheal defects with a complete split of the trachea extending to one main bronchus (21). The child’s symptoms are based on the extent of the cleft. Type I clefts can be asymptomatic, however, high-degree laryngotracheal clefts can lead to stridor, aspiration, coughing, chocking and recurrent pneumonia. Usually, endoscopy is considered as the gold-standard during the work-up. The treatment is dependent on the type of the cleft. In general, laryngeal clefts may be managed endoscopically, laryngotracheal clefts warrant open surgical repair.
Vocal fold paralysis
Vocal fold paralysis in children can have neurologic causes or occur after cardiothoracic procedures. Reported recovery rates vary widely in the literature between 10% and 80% (22-24). As in adults cardiothoracic operations can lead to inadvertent vocal fold paralysis. In the vast majority of cases the left recurrent laryngeal nerve is affected due to its close relationship to the left pulmonary artery and the aortic arch. Immediate treatment is usually not recommended and these children should be followed in 3-month intervals (watchful waiting). Logopedic therapy is hardly possible in small children. If the paralysis persists a voice improving intervention e.g., injection thyroplasty in case of a large glottic gap can be performed (25).
Surgical techniques
Aortopexy
In children with symptomatic, localized tracheomalacia due to compression by the aortic arch or its central takeoffs, aortopexy is the treatment of choice. Additionally, the pulmonary artery, the innominate artery and the pericardium can be suspended, depending on the individual situation. The aim of aortopexy is to de-compress the trachea by lifting the pre-tracheal vessels. Several approaches have been proposed in literature including a right-sided or left-sided anterior thoracotomy, sternotomy or video-assisted thoracoscopic surgery. Typically, the thymus is partially removed to gain space. Afterwards, suspension sutures are placed through the adventitia at the aortic arch and the brachiocephalic artery and tied to the undersurface of the sternum. Alternatively, sutures can also be placed though the aortic pericardial reflecting fold omitting the need of placing sutures directly into the vessel wall (26). The correct placement of the sutures and its tension can be directly visualized by performing a simultaneous bronchoscopy. The success rate of this procedure is above 95 percent with a very low rate of complications in experienced centers (10,27).
Tracheal resection
The surgical technique of tracheal resection and end-to-end anastomosis in children is very similar to adults. The principles of operation involve resection of all the diseased parts of the trachea and approximating healthy mucosa together. Due to the high flexibility of the pediatric airway, extensive mobilization maneuvers (e.g., supraglottic release) are hardly ever necessary and a tensionless anastomosis can be obtained in nearly all cases. The anastomosis is usually performed with a running 5-0 or 6-0 suture for the backwall and interrupted single 5-0 stiches to adapt the cartilaginous parts. We routinely use monofilament absorbable suture material (PDS) (28) but other centers have reported similar outcomes with non-absorbable monofilament (Prolene) or braided absorbable sutures (Vicryl) (3).
Standard cricotracheal resection
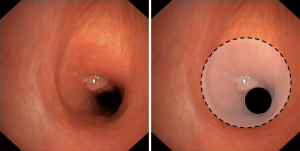
Subglottic stenosis can be corrected by cricotracheal resections (CTR) with excellent results and decannulation rates of over 90 percent (29,30). This standard procedure includes the removal of the cricoid arch, whereas the cricoid plate is left in place. This is the gold standard for anterior stenosis, being mostly the result of a high tracheostomy. If the scar extends towards the cricoid plate, a dorsal mucosectomy can be added. The denudated part of the cricoid plate is then covered by a dorsal mucosa flap of the trachea (Figure 2). There is only a limited use of lateral sub-musectomy and tailored cricoplasty techniques in children, as advocated for adult patients by the Boston group (31). In children with a laterolateral narrowing enlargement techniques such as laryngotracheal reconstructions (LTR) or extended partial cricotracheal resections (pCTR) lead to better results.
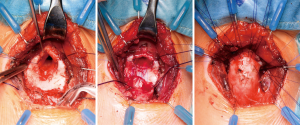
LTR
The main principle of LTR involves the enlargement of the glottic and subglottic diameter by interposition of cartilage grafts. Initial experience of this technique was published by Cotton and colleagues in the 1970s (32). This technique slowly superseded previous laryngoplasty techniques without cartilage grafting such as the Rethi procedure (33). Usually the cartilage is harvested from the costal arch (34), however, others have described the use of the thyroid ala or nasal septal cartilage (35,36). A complete anterior laryngofissure exposes the posterior surface of the cricoid plate. The plate is then divided in the midline and a rib graft with two lateral flanges is snapped into position. The anterior portion of the airway is enlarged in the same way using a second cartilage graft (Figure 3). The technique of LTR leads to excellent results, particularly in mild to moderate stenosis (37). However, in cases with extensive scarring (modified Myer-Cotton grade III and IV), results are less optimal and extended pCTRs should be applied (38).
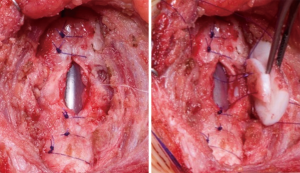
Extended pCTR
The extended pCTR is a combination of resection and reconstruction techniques using rib cartilage grafts for airway enlargement. In addition to a standard cricotracheal resection (with a dorsal mucosectomy), the thyroid is split in the midline for exposure of the posterior glottis. Then the cricoid plate is divided in the midline. If extensive scarring of the interarytenoid muscle or cricoarytenoid joint fixation is found, the division has to be extended cephalad. A rib cartilage graft is then inserted as per the technique described above (LTR). A thyrotracheal anastomosis is performed after trimming the distal end of the trachea. The dorsal cartilage interposition has to be fully covered by a distal mucosal flap. Anteriorly, the thyroid is partially closed in order to reapproximate the anterior commissure and the vocal folds. A wedge of cartilage pedicled to the anterior tracheal wall is trimmed and interpositioned between the lower portion of the thyroid split (Figure 4).
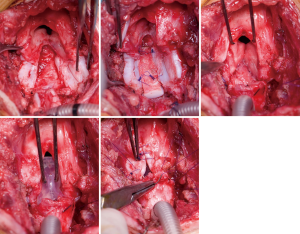
Extended pCTR often requires an endoluminal stabilization since the pediatric airway is still flexible and the reconstruction is usually not immediately stable. Currently, the LT-Mold (Bredam S.A., St. Sulpice, Switzerland), which was developed in Lausanne, is the most distinguished airway-prosthesis for pediatric patients. It is made of soft silicone and therefore avoids pressure necrosis and the formation of granulation tissue (39). The prosthesis was created molding pediatric cadaver larynges and therefore is adapted to anatomical features of the glottic and subglottic region.
A temporary tracheostomy has to be implemented distal to the reconstruction. The LT-Mold can be easily removed endoscopically after 6-8 weeks and patients can be weaned from the tracheostomy thereafter.
To date two major series of extended pCTR for severe laryngotracheal stenosis have been published in the literature. Both groups from Cincinnati and Lausanne report excellent outcomes with decannulation rates of 90% (40) and 94%, respectively (29). Despite the severity of the stenosis, functional outcome is remarkable. During long-term follow-up, 65% of patient had a completely normal breathing pattern, even on exertion. Although, some degree of dysphonia had remained in the majority of children, particularly in cases with preexisting cricoarytenoid joint fixation. Swallowing is hardly ever impaired with normal swallowing reported in 94% of patients (4).
Slide tracheoplasty
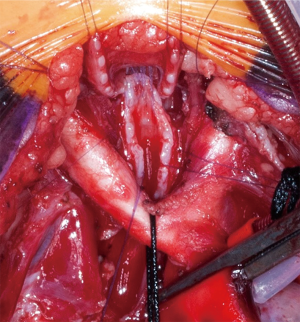
Slide tracheoplasty is a wide-spread technique in pediatric airway surgery. It was originally developed to treat long-segment congenital stenosis and first described in 1989 by Tsang and Goldstraw (41). The basic principle of tracheoplasty is a doubling of the stenotic tracheal lumen by dividing the stenotic airway in the middle of the stenosis and sliding the two newly formed segments into each other (Figure 5). The largest published cohort of slide tracheoplasties is reported by the airway service of the Great Ormond Street Hospital, UK, with a pooled experience of over 100 patients (42). Short-term and long-term results are good (43,44). Patients with preoperative ECMO requirement, long-segment tracheomalacia and extension of the stenosis into the main bronchi experienced worse postoperative outcomes (42). About 50% of long-segment congenital stenosis patients present with a concomitant cardiovascular anomaly. A combined repair of the tracheal stenosis and the cardiovascular malformation is required, necessitating cardiopulmonary bypass. If there is no concomitant defect, the operation can also be performed using ECMO for maintaining oxygenation (45). Due to the excellent results of slide tracheoplasties some centers have expanded their indication to children with intermediate and even short-segment stenosis (43). A resection of greater than 30 percent of the airway length is associated with an increased risk of anastomotic complications (3). Thus, the technique of slide tracheoplasty poses an interesting alternative to mere resection and end-to-end anastomosis.
Conclusions
Pediatric airway surgery is a challenging field of medicine, which involves a dedicated multidisciplinary team. Thoracic surgeons, ENT surgeons, phoniatricians, pediatricians and anesthetists are needed to treat these often complex patients. In experienced hands excellent short- and long-term results can be achieved, thus, a centralization of these highly demanding patients should be considered.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Eckel HE, Koebke J, Sittel C, et al. Morphology of the human larynx during the first five years of life studied on whole organ serial sections. Ann Otol Rhinol Laryngol 1999;108:232-8. [Crossref] [PubMed]
- Masaoka A, Yamakawa Y, Niwa H, et al. Pediatric and adult tracheobronchomalacia. Eur J Cardiothorac Surg 1996;10:87-92. [Crossref] [PubMed]
- Wright CD, Graham BB, Grillo HC, et al. Pediatric tracheal surgery. Ann Thorac Surg 2002;74:308-13; discussion 314. [Crossref] [PubMed]
- Monnier P. Pediatric airway surgery management of laryngotracheal stenosis in infants and children. 2011:xvii, 371 p.
- Walner DL, Loewen MS, Kimura RE. Neonatal subglottic stenosis--incidence and trends. Laryngoscope 2001;111:48-51. [Crossref] [PubMed]
- Overman AE, Liu M, Kurachek SC, et al. Tracheostomy for infants requiring prolonged mechanical ventilation: 10 years' experience. Pediatrics 2013;131:e1491-6. [Crossref] [PubMed]
- Monnier P, Ikonomidis C, Jaquet Y, et al. Proposal of a new classification for optimising outcome assessment following partial cricotracheal resections in severe pediatric subglottic stenosis. Int J Pediatr Otorhinolaryngol 2009;73:1217-21. [Crossref] [PubMed]
- Bennett JD. High tracheostomy and other errors--revisited. J Laryngol Otol 1996;110:1003-7. [Crossref] [PubMed]
- Bové T, Demanet H, Casimir G, et al. Tracheobronchial compression of vascular origin. Review of experience in infants and children. J Cardiovasc Surg (Torino) 2001;42:663-6. [PubMed]
- Dave S, Currie BG. The role of aortopexy in severe tracheomalacia. J Pediatr Surg 2006;41:533-7. [Crossref] [PubMed]
- Oshima Y, Yamaguchi M, Yoshimura N, et al. Management of pulmonary artery sling associated with tracheal stenosis. Ann Thorac Surg 2008;86:1334-8. [Crossref] [PubMed]
- Backer CL, Mavroudis C, Gerber ME, et al. Tracheal surgery in children: an 18-year review of four techniques. Eur J Cardiothorac Surg 2001;19:777-84. [Crossref] [PubMed]
- Kirshbom PM, Jaggers JJ, Ungerleider RM. Tetralogy of fallot with absent pulmonary valve: simplified technique for homograft repair. J Thorac Cardiovasc Surg 1999;118:1125-7. [Crossref] [PubMed]
- Serio P, Nenna R, Fainardi V, et al. Residual tracheobronchial malacia after surgery for vascular compression in children: treatment with stentingdagger. Eur J Cardiothorac Surg 2016. [Epub ahead of print]. [Crossref]
- Pillai JB, Smith J, Hasan A, et al. Review of pediatric airway malacia and its management, with emphasis on stenting. Eur J Cardiothorac Surg 2005;27:35-44. [Crossref] [PubMed]
- Gross RE, Piotti E. The surgery of infancy and childhood: its principles and techniques. Philadelphia: Saunders, 1953:xxiv, 1000.
- Kovesi T, Rubin S. Long-term complications of congenital esophageal atresia and/or tracheoesophageal fistula. Chest 2004;126:915-25. [Crossref] [PubMed]
- Healey PJ, Sawin RS, Hall DG, et al. Delayed primary repair of esophageal atresia with tracheoesophageal fistula: is it worth the wait? Arch Surg 1998;133:552-6. [Crossref] [PubMed]
- Hirschl RB, Yardeni D, Oldham K, et al. Gastric transposition for esophageal replacement in children: experience with 41 consecutive cases with special emphasis on esophageal atresia. Ann Surg 2002;236:531-9; discussion 539-41. [Crossref] [PubMed]
- Moungthong G, Holinger LD. Laryngotracheoesophageal clefts. Ann Otol Rhinol Laryngol 1997;106:1002-11. [Crossref] [PubMed]
- Benjamin B, Inglis A. Minor congenital laryngeal clefts: diagnosis and classification. Ann Otol Rhinol Laryngol 1989;98:417-20. [Crossref] [PubMed]
- Pereira KD, Webb BD, Blakely ML, et al. Sequelae of recurrent laryngeal nerve injury after patent ductus arteriosus ligation. Int J Pediatr Otorhinolaryngol 2006;70:1609-12. [Crossref] [PubMed]
- Jabbour J, Martin T, Beste D, et al. Pediatric vocal fold immobility: natural history and the need for long-term follow-up. JAMA Otolaryngol Head Neck Surg 2014;140:428-33. [Crossref] [PubMed]
- de Gaudemar I, Roudaire M, Francois M, et al. Outcome of laryngeal paralysis in neonates: a long term retrospective study of 113 cases. Int J Pediatr Otorhinolaryngol 1996;34:101-10. [Crossref] [PubMed]
- Patel NJ, Kerschner JE, Merati AL. The use of injectable collagen in the management of pediatric vocal unilateral fold paralysis. Int J Pediatr Otorhinolaryngol 2003;67:1355-60. [Crossref] [PubMed]
- Köylüoğlu G, Gunay I, Ceran C, et al. Pericardial flap aortopexy: an easy and safe technique in the treatment of tracheomalacia. J Cardiovasc Surg (Torino) 2002;43:295-7. [PubMed]
- Jennings RW, Hamilton TE, Smithers CJ, et al. Surgical approaches to aortopexy for severe tracheomalacia. J Pediatr Surg 2014;49:66-70; discussion 70-1. [Crossref] [PubMed]
- Hoetzenecker K, Schweiger T, Schwarz S, et al. Summarized institutional experience of paediatric airway surgerydagger. Eur J Cardiothorac Surg 2016;49:1119-26. [Crossref] [PubMed]
- White DR, Cotton RT, Bean JA, et al. Pediatric cricotracheal resection: surgical outcomes and risk factor analysis. Arch Otolaryngol Head Neck Surg 2005;131:896-9. [Crossref] [PubMed]
- Cotton RT. Management of subglottic stenosis. Otolaryngol Clin North Am 2000;33:111-30. [Crossref] [PubMed]
- Liberman M, Mathisen DJ. Tailored cricoplasty: an improved modification for reconstruction in subglottic tracheal stenosis. J Thorac Cardiovasc Surg 2009;137:573-8; discussion 578-9. [Crossref] [PubMed]
- Fearon B, Cotton R. Surgical correction of subglottic stenosis of the larynx in infants and children. Progress report. Ann Otol Rhinol Laryngol 1974;83:428-31. [Crossref] [PubMed]
- Réthi A. An operation for cicatricial stenosis of the larynx. J Laryngol Otol 1956;70:283-93. [Crossref] [PubMed]
- Jaquet Y, Lang F, Pilloud R, et al. Partial cricotracheal resection for pediatric subglottic stenosis: long-term outcome in 57 patients. J Thorac Cardiovasc Surg 2005;130:726-32. [Crossref] [PubMed]
- Forte V, Chang MB, Papsin BC. Thyroid ala cartilage reconstruction in neonatal subglottic stenosis as a replacement for the anterior cricoid split. Int J Pediatr Otorhinolaryngol 2001;59:181-6. [Crossref] [PubMed]
- Drettner B, Lindholm CE. Experimental tracheal reconstruction with composite graft from nasal septum. Acta Otolaryngol 1970;70:401-7. [Crossref] [PubMed]
- Hartnick CJ, Hartley BE, Lacy PD, et al. Surgery for pediatric subglottic stenosis: disease-specific outcomes. Ann Otol Rhinol Laryngol 2001;110:1109-13. [Crossref] [PubMed]
- Ochi JW, Evans JN, Bailey CM. Pediatric airway reconstruction at Great Ormond Street: a ten-year review. I. Laryngotracheoplasty and laryngotracheal reconstruction. Ann Otol Rhinol Laryngol 1992;101:465-8. [Crossref] [PubMed]
- Alshammari J, Monnier P. Airway stenting with the LT-Mold for severe glotto-subglottic stenosis or intractable aspiration: experience in 65 cases. Eur Arch Otorhinolaryngol 2012;269:2531-8. [Crossref] [PubMed]
- George M, Ikonomidis C, Jaquet Y, et al. Partial cricotracheal resection in children: potential pitfalls and avoidance of complications. Otolaryngol Head Neck Surg 2009;141:225-31. [Crossref] [PubMed]
- Tsang V, Murday A, Gillbe C, et al. Slide tracheoplasty for congenital funnel-shaped tracheal stenosis. Ann Thorac Surg 1989;48:632-5. [Crossref] [PubMed]
- Butler CR, Speggiorin S, Rijnberg FM, et al. Outcomes of slide tracheoplasty in 101 children: a 17-year single-center experience. J Thorac Cardiovasc Surg 2014;147:1783-9. [Crossref] [PubMed]
- Rutter MJ, Cotton RT, Azizkhan RG, et al. Slide tracheoplasty for the management of complete tracheal rings. J Pediatr Surg 2003;38:928-34. [Crossref] [PubMed]
- Manning PB, Rutter MJ, Border WL. Slide tracheoplasty in infants and children: risk factors for prolonged postoperative ventilatory support. Ann Thorac Surg 2008;85:1187-91; discussion 1191-2. [Crossref] [PubMed]
- Huang SC, Wu ET, Chi NH, et al. Perioperative extracorporeal membrane oxygenation support for critical pediatric airway surgery. Eur J Pediatr 2007;166:1129-33. [Crossref] [PubMed]


