Hypercapnia during acute respiratory distress syndrome: the tree that hides the forest!
Introduction
Nin and coworkers recently published an article that examines whether hypercapnia has an impact on mortality in patients with moderate or severe acute respiratory distress syndrome (ARDS) (1). To answer this important question, they conducted a post-hoc analysis of three prospective non-interventional international cohort studies focusing on ARDS (2-4). Among the 18,302 mechanically ventilated patients included (2-4), 1,899 were ventilated for more than 24 h because of ARDS or developed ARDS after the first 24 h of mechanical ventilation (1). The study population was exclusively composed of patients with moderate or severe ARDS according to the Berlin definition (5). Severe hypercapnia was defined as a highest PaCO2 within 48 h after the diagnosis of ARDS ≥50 mmHg (≥85th percentile of the distribution of PaCO2 values). The main result of the study is that severe hypercapnia is associated with higher ICU mortality and more organ failure (including hemodynamic failure), even after adjustment for possible confounders such as age, SAPS II, PaO2/FiO2, positive end-expiratory pressure (PEEP), driving pressure, respiratory rate (RR), acidosis, corrected minute ventilation (a surrogate of dead space), the use of pressure/volume limitation strategy (PLS) and the period of the study. The authors also nicely reported that the incidence of severe hypercapnia increased significantly with the time period (1998, 2004 and 2010) (1), as a consequence of different respiratory strategies and the feeling of many intensivists that hypercapnia is beneficial (6). Regarding the association between severe hypercapnia and hemodynamic failure, it is of note that the occurrence of sepsis did not differ between patients with or without severe hypercapnia (1), suggesting that it could be related to the deleterious effects of PaCO2 on the pulmonary circulation and the right ventricle. This article by Nin and coworkers strongly suggests that hypercapnia can no longer be considered as a supporting or a therapeutic factor in ARDS, but more as a (deleterious) consequence of the respiratory strategy that should be limited. This is what we discuss here.
The impact of hypercapnia in ARDS
The impact of hypercapnia has not been clearly elucidated and is still controversial given its disparate effects (7,8). Nevertheless, it has come to be seen as a major factor in ARDS and its pivotal role has emerged from the historical evolution of ventilator strategies (Figure 1). During the first 20 years following the characterization of ARDS, the ventilatory strategy relied on “high” tidal volume to normalize PaCO2; hypercapnia almost did not exist. Carbon dioxide (CO2) has become a noticeable factor since the early 1990s with the work from Hickling et al., who demonstrated in two observational uncontrolled studies that mechanical ventilation with low volume and low pressure, along with an increase in PaCO2, was associated with better outcome (9,10). The concept of “permissive hypercapnia” was born (10). At that time, this new paradigm was strengthened by experimental studies demonstrating the beneficial effects of CO2 on inflammation (11), even though the effect of hypercapnia in the immuno-inflammatory response to sepsis remains controversial (11-13). More than 10 years of “permissive hypercapnia” ventilatory strategy even led some authors to propose the concept of “therapeutic hypercapnia” (6). Nevertheless, more recent studies have tempered such an enthusiasm by demonstrating harmful effects of CO2 on the lung on the one hand (14,15), and on the pulmonary circulation and the right ventricle on the other hand (16,17).
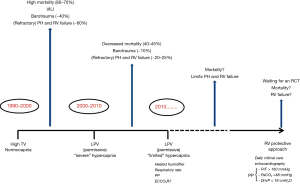
Effect of CO2 on the pulmonary circulation and the right ventricle
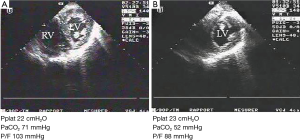
The effects of CO2 on the circulatory system are multimodal and can be schematically classified in three categories: a depression of the left ventricle contraction secondary to an acute increase in PaCO2 responsible for intra-cellular acidosis (18), an increase in cardiac output relying on a peripheral reduced vasomotor tone which may counterbalance the depressant effect of CO2 on the left ventricle (19), and finally an increase in right ventricular afterload linked to pulmonary arterial vasoconstriction (20,21). In ARDS, the main effect of hypercapnia is exerted on the right ventricle, because of the huge increase in pulmonary arterial resistance, leading to right ventricular failure, named acute cor pulmonale (ACP) (22). Pulmonary arterial hypertension is constant in ARDS and was observed before the area of permissive hypercapnia, as the consequence of the high transpulmonary pressure, which compresses the pulmonary capillaries (23), and alteration of the capillary pulmonary circulation mediated by inflammatory and procoagulant phenomena (24,25). The speed of CO2 augmentation probably plays an important role in the occurrence of right ventricular failure. Mekontso-Dessap et al. showed that an increase in PaCO2 to around 70 mmHg although associated with a decrease in tidal volume and in driving pressure, was responsible for right ventricular failure (17) (Figure 2). Two specific points were discussed in this study. First, the short time course (no more than 1.5 h) of PaCO2 changes. Whether a more progressive increase in PaCO2 could be responsible for the same right ventricular failure remains to be elucidated. Second, the study did not differentiate between the effects of hypercapnia itself and the induced respiratory acidosis. Interestingly, the effect of severe hypercapnia in the study by Nin et al. was independent of pH (1) .
Effects of PaCO2 on mortality: making the link with the right ventricle
Many recent studies suggest that right ventricular protection is at the core of the effect of different ventilatory strategies on prognosis. Pulmonary hypertension and pulmonary vascular dysfunction have been shown to be associated with poor outcome in ARDS (26). Lhéritier et al. demonstrated that a PaCO2 >60 mmHg was an independent risk factor for ACP and this was strongly confirmed by Mekontso-Dessap et al. in a large cohort of 752 patients with moderate or severe ARDS. In this latter study, a PaCO2 ≥48 mmHg, a value very close to that reported by Nin et al., was an independent risk factor for ACP with an odds ratio of 2.9 and severe ACP was independently associated with hospital mortality in moderate to severe ARDS (27). Severe hypercapnia is associated with more circulatory failure not related to sepsis, and with higher ICU mortality (1). Interestingly, prone position, a strategy well known to decrease PaCO2 (28) and to improve right ventricular function (29), has been reported to improve prognosis (30).
How to protect the right ventricle from hypercapnia
Based on this literature analysis, we can suggest that control of permissive hypercapnia during the PLS may come to be at the core of the ventilatory strategy. It has been suggested that the positive results of the ARMA study (31) could at least in part be explained by the effort to correct hypercapnia by increasing RR (32,33). Nevertheless, high RR exposes ARDS patients to the risk of dynamic hyperinflation, which dramatically impairs right ventricular ejection (34). This is why the RR must be cautiously increased by verifying with a 4-second end-expiratory pause that no intrinsic PEEP has been generated (35). To avoid such a side effect of too high a RR, Prin et al. reported that the use of heated humidifiers significantly decreased the level of PaCO2 in moderate or severe ARDS, as compared with the use of heat and moisture exchangers (36). Aguirre-Bermeo et al. recently demonstrated in 13 ARDS patients that end-inspiratory pause prolongation from 0.1–0.2 to 0.7 s induced a significant decrease in PaCO2 from 54±9 to 50±8 mmHg (37). They showed that this decrease in PaCO2 was correlated with the drop in physiological dead space (37). However, it has also been reported that ventilation with inverted I/E ratio could have deleterious hemodynamic effects (38). As briefly discussed above, prone position is another very pertinent way to limit hypercapnia and to increase oxygenation without increasing PEEP, tidal volume and RR, by recruiting the lung and decreasing its heterogeneity (28,39). Even though this was not reported in the study by Nin et al., it is key to improving both PaCO2 and PaO2/FiO2, since hypoxia may magnify the deleterious effect of hypercapnia on the pulmonary circulation (40). There are preliminary data on the use of extracorporeal CO2 removal (ECCOR) to limit hypercapnia and then to alleviate right ventricular overload by controlling the CO2 level. In an experimental model of ARDS conducted in pigs, veno-venous CO2 removal therapy enabled protective ventilation while maintaining normocapnia, with decreased pulmonary hypertension, and improved right ventricular function (41). Whether such approach, combining a lung protective (or even ultra protective) approach and ECCOR, could improve right ventricular function or prevent right ventricular failure remains to be studied, even though it may probably be applied in critical situations (42).
It is high time to evaluate the right ventricular protective approach
Nowadays, the right ventricle is definitively the weak link in ARDS and one of the main objectives of management is to prevent right ventricular failure or to support right ventricular function. The right ventricular protective strategy has recently been proposed (43) and relies mostly on the control of right ventricular overload, by controlling hypoxemia, driving pressure and hypercapnia (44). The cornerstone of such a strategy is probably the early and prolonged use of prone position for the most severe cases in terms of oxygenation (30) or for patients who develop right ventricular failure despite lung protective ventilation. In this strategy, severe hypercapnia, as defined by Nin et al., has to be avoided, PaO2/FiO2 optimized and driving pressure decreased. PaCO2 is controlled and maintained <48–50 mmHg with cautious increases in RR, while checking that no intrinsic PEEP has been generated, and with the use of heated humidifiers. During the whole ventilator support of such patients, the right ventricle has to be rigorously monitored by at least daily critical care echocardiography during the first 3 days, in order to detect early right ventricular dysfunction, which would motivate adaptation of treatment. Based on the cumulative information that the right ventricle is at the core of respiratory strategy, the right ventricular protective approach has yet to be evaluated in a randomized control trial.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nin N, Muriel A, Peñuelas O, et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med 2017;43:200-8. [Crossref] [PubMed]
- Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002;287:345-55. [Crossref] [PubMed]
- Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med 2008;177:170-7. [Crossref] [PubMed]
- Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188:220-30. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill—too little of a good thing? Lancet 1999;354:1283-6. [Crossref] [PubMed]
- Beitler JR, Hubmayr RD, Malhotra A. CrossTalk opposing view: there is not added benefit to providing permissive hypercapnia in the treatment of ARDS. J Physiol 2013;591:2767-9. [Crossref] [PubMed]
- Curley GF, Laffey JG, Kavanagh BP. CrossTalk proposal: there is added benefit to providing permissive hypercapnia in the treatment of ARDS. J Physiol 2013;591:2763-5. [Crossref] [PubMed]
- Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 1990;16:372-7. [Crossref] [PubMed]
- Hickling KG, Walsh J, Henderson S, et al. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 1994;22:1568-78. [Crossref] [PubMed]
- Laffey JG, Tanaka M, Engelberts D, et al. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med 2000;162:2287-94. [Crossref] [PubMed]
- Broccard AF, Hotchkiss JR, Vannay C, et al. Protective effects of hypercapnic acidosis on ventilator-induced lung injury. Am J Respir Crit Care Med 2001;164:802-6. [Crossref] [PubMed]
- Sinclair SE, Kregenow DA, Lamm WJ, et al. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med 2002;166:403-8. [Crossref] [PubMed]
- Doerr CH, Gajic O, Berrios JC, et al. Hypercapnic acidosis impairs plasma membrane wound resealing in ventilator-injured lungs. Am J Respir Crit Care Med 2005;171:1371-7. [Crossref] [PubMed]
- Jaitovich A, Angulo M, Lecuona E, et al. High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific Ring finger protein 1 (MuRF1). J Biol Chem 2015;290:9183-94. [Crossref] [PubMed]
- Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med 2013;39:1725-33. [Crossref] [PubMed]
- Mekontso Dessap A, Charron C, Devaquet J, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med 2009;35:1850-8. [Crossref] [PubMed]
- Jerusalem E, Starling EH. On the significance of carbon dioxide for the heart beat. J Physiol 1910;40:279-94. [Crossref] [PubMed]
- Brofman JD, Leff AR, Munoz NM, et al. Sympathetic secretory response to hypercapnic acidosis in swine. J Appl Physiol 1990;69:710-7. [PubMed]
- Viitanen A, Salmenperä M, Heinonen J. Right ventricular response to hypercarbia after cardiac surgery. Anesthesiology 1990;73:393-400. [Crossref] [PubMed]
- Carvalho CR, Barbas CS, Medeiros DM, et al. Temporal hemodynamic effects of permissive hypercapnia associated with ideal PEEP in ARDS. Am J Respir Crit Care Med 1997;156:1458-66. [Crossref] [PubMed]
- Jardin F, Dubourg O, Bourdarias JP. Echocardiographic pattern of acute cor pulmonale. Chest 1997;111:209-17. [Crossref] [PubMed]
- Whittenberger JL, McGregor M, Berglund E, et al. Influence of state of inflation of the lung on pulmonary vascular resistance. J Appl Physiol 1960;15:878-82. [PubMed]
- Zapol WM, Kobayashi K, Snider MT, et al. Vascular obstruction causes pulmonary hypertension in severe acute respiratory failure. Chest 1977;71:306-7. [Crossref] [PubMed]
- Price LC, McAuley DF, Marino PS, et al. Pathophysiology of pulmonary hypertension in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2012;302:L803-15. [Crossref] [PubMed]
- Bull TM, Clark B, McFann K, et al. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med 2010;182:1123-8. [Crossref] [PubMed]
- Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862-70. [Crossref] [PubMed]
- Charron C, Repessé X, Bouferrache K, et al. PaCO2 and alveolar dead space are more relevant than PaO2/FiO2 ratio in monitoring the respiratory response to prone position in ARDS patients: a physiological study. Crit Care 2011;15:R175. [Crossref] [PubMed]
- Vieillard-Baron A, Charron C, Caille V, et al. Prone positioning unloads the right ventricle in severe ARDS. Chest 2007;132:1440-6. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Slutsky AS, Ranieri VM. Mechanical ventilation: lessons from the ARDSNet trial. Respir Res 2000;1:73-7. [Crossref] [PubMed]
- Tobin MJ. Culmination of an era in research on the acute respiratory distress syndrome. N Engl J Med 2000;342:1360-1. [Crossref] [PubMed]
- Vieillard-Baron A, Prin S, Augarde R, et al. Increasing respiratory rate to improve CO2 clearance during mechanical ventilation is not a panacea in acute respiratory failure. Crit Care Med 2002;30:1407-12. [Crossref] [PubMed]
- Pepe PE, Marini JJ. Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction: the auto-PEEP effect. Am Rev Respir Dis 1982;126:166-70. [PubMed]
- Prin S, Chergui K, Augarde R, et al. Ability and safety of a heated humidifier to control hypercapnic acidosis in severe ARDS. Intensive Care Med 2002;28:1756-60. [Crossref] [PubMed]
- Aguirre-Bermeo H, Moran I, Bottiroli M, et al. End-inspiratory pause prolongation in acute respiratory distress syndrome patients: effects on gas exchange and mechanics. Ann Intensive Care 2016;6:81. [Crossref] [PubMed]
- Mercat A, Titiriga M, Anguel N, et al. Inverse ratio ventilation (I/E = 2/1) in acute respiratory distress syndrome: a six-hour controlled study. Am J Respir Crit Care Med 1997;155:1637-42. [Crossref] [PubMed]
- Vieillard-Baron A, Rabiller A, Chergui K, et al. Prone position improves mechanics and alveolar ventilation in acute respiratory distress syndrome. Intensive Care Med 2005;31:220-6. [Crossref] [PubMed]
- Enson Y, Giuntini C, Lewis ML, et al. The Influence of Hydrogen Ion Concentration and Hypoxia on the Pulmonary Circulation. J Clin Invest 1964;43:1146-62. [Crossref] [PubMed]
- Morimont P, Guiot J, Desaive T, et al. Veno-venous extracorporeal CO2 removal improves pulmonary hemodynamics in a porcine ARDS model. Acta Anaesthesiol Scand 2015;59:448-56. [Crossref] [PubMed]
- Vieillard-Baron A, Matthay M, Teboul JL, et al. Experts' opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 2016;42:739-49. [Crossref] [PubMed]
- Vieillard-Baron A, Price LC, Matthay MA. Acute cor pulmonale in ARDS. Intensive Care Med 2013;39:1836-8. [Crossref] [PubMed]
- Repessé X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest 2015;147:259-65. [Crossref] [PubMed]


