Decrease in performance status after lobectomy mean poor prognosis in elderly lung cancer patients
Introduction
The incidence of lung cancer is increasing in the older population. In cases of resectable primary lung cancer, surgery remains the best therapeutic option for obtaining cure, irrespective of the patient’s age. Lobectomy is still regarded as the best surgical procedure for patients with non-small cell lung cancer (NSCLC) (1). However, elderly patients may have high risk of decreased performance status (PS) after lobectomy, and we think that decreased PS may result in poor prognosis in elderly lung cancer patients. The presumed risk of decreased PS after lobectomy may result in the selection of sub-optimal surgeries, such as limited resections (2,3). We believe that, depending on their functional status, all elderly cancer patients should be offered the optimal treatment at any age. To improve surgical decision making for elderly patients with lung cancer, surgeons should carefully explain both the survival benefits of lobectomy and the changes in PS after lobectomy.
This study was performed to identify the association between PS after lobectomy and prognosis in elderly patients with lung cancer.
Methods
Institutional review board approval was obtained for this study and we waived the requirement for patient consent because of the retrospective nature of this study. This study was a retrospective review of 137 patients aged at least 75 years who underwent lobectomy for NSCLC from January 2004 through December 2014. Our surgical policy for patients with resectable lung cancer was lobectomy with mediastinal lymph node dissection (MLD). However, patients who were demonstrated to lack mediastinal lymphadenopathy (via computed tomography) and hilar disease (via intraoperative rapid diagnosis) were allowed to omit MLD.
The routine preoperative assessments included a medical history, a physical examination, basic blood tests, an electrocardiogram, and a pulmonary function test. All intraoperative and postoperative events were recorded. Operative mortality included both deaths that occurred within 30 days after the operation and later deaths that occurred during the same hospitalization. Morbidity was defined as the occurrence of at least one postoperative event.
Clinical staging was based on computed tomography of the chest and abdomen, brain computed tomography, or magnetic resonance imaging, radionuclide bone scan, and/or positron emission tomography with fluorine-18 fluorodeoxyglucose. Mediastinal and hilar lymph node status was defined as positive if the chest computed tomography showed that the shorter axis of any node was greater than 1 cm. Mediastinoscopy and endobronchial ultrasound guided-biopsy were not routinely performed.
Patients who had suspected lung cancer underwent preoperative PS assessments by asking to them at the time of hospital admission for surgery. In the same way, patients received lobectomy underwent postoperative PS assessments at 6-month after surgery in principle. However, 3 patients assessed postoperative PS at 1 month because of death (1 patient) and changing hospital (2 patients), 4 patients at 2 month because of death (3 patients) and changing hospital (1 patient), 1 patient at 4-month because of changing hospital. Preoperative and postoperative PS were based on the Eastern Cooperative Oncology Group scores (4) that were included in our database. Patients were classified into 2 groups based on their PS results: patients whose postoperative PS was the same as preoperative PS were group 1, while patients whose postoperative PS decreased relative to preoperative PS were group 2.
We compared the characteristics and survival outcomes of group 1 and group 2 patients. Univariate analyses were performed to investigate between-group differences in preoperative and intraoperative factors, including the following 13 clinical characteristics: sex, age, preoperative PS, number of comorbidities, presence of chronic obstructive pulmonary disease, presence of restrictive ventilatory impairment, history of ischemic heart disease, presence of diabetes, clinical stage, surgical approach (thoracotomy vs. thoracoscopy), MLD, histology of adenocarcinoma, and histology of squamous cell carcinoma. The statistical analysis was performed using SPSS statistical software (version 22, SPSS, Inc., Chicago, IL, USA). Survival was estimated using the Kaplan-Meier method, and dichotomous variables were compared using the χ2 test. Results were considered statistically significant for P≤0.05.
Results
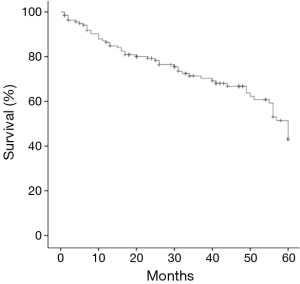
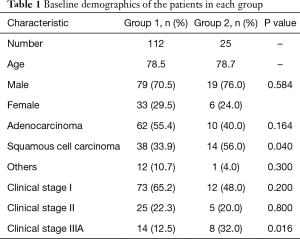
During the study period, 679 patients underwent lung resection for NSCLC in our hospital. In these, 463 patients (68.2%) received lobectomy, and 137 patients (20.2%) were aged 75 years or older (median age 78.5 years, range, 75 to 88 years). Their demographics are summarized in Table 1. Twenty-two patients omitted MLD based on the judgments of their primary surgeons. In the entire study cohort, the 5-year survival rate was 43.1% and the median survival time was 45.6 months (Figure 1). The median duration of follow-up was 36.3 months. Postoperative mortality occurred in 4 (2.9%) patients, but there was no mortality within 30 postoperative days. Postoperative morbidity occurred in 46 (33.6%) patients. The causes of morbidity were pneumonia (n=15), cardiac arrhythmia (n=14), prolonged pulmonary fistula (over 7 days or induction of pleurodesis; n=12), delirium (n=6), and other conditions (n=11).
Full table
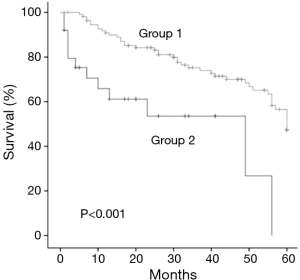
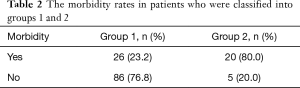
Of the 137 patients in our study, 112 were classified into group 1 and 25 were classified into group 2. The patients in Group 1 (79 men and 33 women) had a mean age of 78.5 years. Sixty-two of them had adenocarcinomas, 32 had squamous cell carcinomas, and 12 had other tumors. The patients had clinical stage I (n=73), II (n=25), or IIIA (n=14) disease. The 5-year survival rate was 47.4% and the median survival time was 48.0 months (Figure 2). Postoperative morbidities occurred in 26 (23.2%) of the patients. The causes of morbidity were pneumonia (n=6), cardiac arrhythmia (n=8), prolonged pulmonary fistula (n=7), delirium (n=4), and other conditions (n=6).
The patients in group 2 (19 men and 6 women) had a mean age of 78.7 years. Ten of these patients had adenocarcinomas, 14 had squamous cell carcinomas, and 1 had another tumor. The patients had clinical stage I (n=12), II (n=5), or IIIA (n=8) disease. The 5-year survival rate was 0% and median survival time was 32.4 months (Figure 2). Postoperative morbidities occurred in 20 (80.0%) patients. The causes of morbidity were pneumonia (n=9), cardiac arrhythmia (n=6), prolonged pulmonary fistula (n=5), delirium (n=2), and other conditions (n=5). In group 2, PS decreased 1 point in 18 patients, 2 points in 3 patients, and 3 points in 4 patients. The 5-year survival rate was significantly higher in group 1 than in group 2 (P<0.001; Figure 2). The morbidity rate was significantly related to postoperative reduction in PS (P<0.001) (Table 2).
Full table
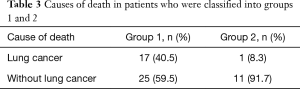
Although lung cancer was main cause of death in group 1, other causes (such as pneumonia) were the main cause of death in group 2 (Table 3) during the postoperative course (P=0.04). In group 1, the cause of death was lung cancer in 17 patients (40.5%), pneumonia in 9 patients (21.4%), other cancers in 3 patients (7.1%), cerebrovascular and cardiovascular events in 2 patients (4.8%), and other conditions or events in 11 patients (26.2%). In group 2, the cause of death was lung cancer in only 1 patient (8.3%), pneumonia in 6 patients (50.0%), another cancer in 1 patient (8.3%), cerebrovascular and cardiovascular events in 1 patient (8.3%), strangulated ileus in 1 patient (8.3%), and unclear in 2 patients (16.6%).
Full table
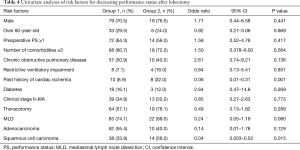
The results of our univariate analyses are listed in Table 4. History of cardiac ischemia (P=0.001) and squamous cell carcinoma (P=0.015) were significantly associated with postoperatively decreased PS. MLD was also associated with postoperatively decreased PS, but the relationship was not statistically significant (P=0.080). There were no statistically significant differences between the two groups in terms of sex, age, preoperative PS, number of comorbidities, presence of chronic obstructive pulmonary disease, presence of restrictive ventilatory impairment, presence of diabetes, history of gastrectomy, surgical approach, or histology of adenocarcinoma.
Full table
Discussion
The findings of this study demonstrate that lobectomy could be performed in patients aged 75 years and older with acceptable survival (5-year survival: 43.1%), morbidity (33.6%), and in-hospital mortality (2.9%) outcomes. These results fall within the ranges of previously published values from other multi-institution and single-institution series (5-9), which have demonstrated 35.5% to 53.1% 5-year survival rates, 38.0% to 58.8% morbidity rates, and 1.6% to 9.0% in-hospital mortality rates. Recent studies have shown promising data regarding surgical and survival outcomes in the elderly (6-10). In addition, a previous evaluation found no statistically significant differences between the rates of postoperative complications in younger and elderly groups (2). Age should not be the sole determinant when considering surgery as a treatment option for cancer in elderly patients.
We believe that the maintenance of life quality (including activities of daily living, quality of life, and PS) is a very important issue in elderly patients. Decreased involvement in activities of daily living is also associated with mortality in the general population (11,12). Although the mechanisms behind the association of physical activity and mortality remain uncertain, we speculate that mortality may be influenced by underlying health problem that remain present in patients, including dementia, comorbid conditions, “frailty” weakness, and decreased bone density (12). A few reports on patients receiving operations for lung cancer have also shown that postoperative quality of life worsened significantly as compared with preoperative baseline values (13,14). It is well known that PS is easier to assess than other instruments. However, no reports have presented information on postoperative PS in elderly patients who have undergone lobectomy for lung cancer. Therefore, we used PS to assess postoperative physical condition. In our study, PS was reduced by 18.2% in elderly patients after lobectomy. We compared group 1 to group 2 in terms of survival, and found that the overall 5-year survival rate was 47.4% in group 1 and 0% in group 2 (P<0.001). In group 1, the main cause of death was lung cancer; however, in group 2, the main causes of death were other conditions and events, such as bacterial pneumonia, interstitial pneumonia, and cardiovascular events. Based on these results, we hypothesize that elderly patients with decreased PS after lobectomy do not have enough reserve capacity, and are therefore more susceptible to other diseases, such as pneumonia. Once they become ill, recovery from these diseases may also be especially difficult for these patients. As a result, they die before tumor recurrence.
The present study demonstrated that history of cardiac ischemia and histology of squamous cell carcinoma were significant risk factors for postoperative reduction in PS. History of cardiac ischemia independently predicted decreasing postoperative PS in a multivariate analysis (P=0.001). Ambrogi et al. reported that cardiovascular comorbidity (including coronary artery disease but not hypertension) appears to have a significant, negative effects on survival and morbidity in patients who have undergone surgery (7). If the cardiovascular diseases are multifocal and major resection (lobectomy or pneumonectomy) has been performed, then the risk of postoperative mortality and morbidity appears to increase further (7). Moreover, during their postoperative courses, many patients die from cardiovascular diseases. Another study reported that patients with cardiovascular comorbidity (including hypertension) did not show increased risks of postoperative mortality and morbidity, and that cardiovascular morbidity did not influence long-term survival (15). However, hypertension was the most common comorbidity in that study, and more limited resections were performed in patients with cardiovascular comorbidity than in patients without cardiovascular comorbidity. Because elderly patients with ischemic heart disease may be susceptible to damage by hypoxic stress, it is easy for them to become exhausted after lobectomy and experience postoperative decreases in PS. Therefore, elderly patients with cardiovascular comorbidity are a high risk group for surgery. The effects of cardiovascular comorbidity should not be underestimated, and thorough preoperative assessments may be valuable for improving prognosis in elderly patients with lung cancer.
Squamous cell carcinoma was independently predictive of postoperatively decreased PS in our multivariate analysis (P=0.015). Several other researchers have previously reported the same finding (2,16). In our study, the frequency of bronchoplasty, angioplasty and composite resection of the thoracic wall was higher in patients with squamous cell carcinoma (17%) than in the other patients (4%). In addition, advanced clinical stage disease (II or IIIA) was more common in patients with squamous cell carcinoma (53.7%) than in the other patients (26.5%). Therefore, surgery for squamous cell carcinoma required longer operative durations and resulted in greater amounts of bleeding than did surgery for the other cancers. As a result, squamous cell carcinoma was a risk factor for morbidity and decreased PS.
Incidence of postoperative complication may predict decreasing postoperative PS. It is also well known that postoperative morbidity has a considerable effect on life quality in patients with lung cancer (2). In our study, the postoperative complication rate was 23.2% in group 1 and 80.0% in group 2 (P<0.001).
The main limitation of the present study was its retrospective nature. Our surgical policy for elderly patients with lung cancer has been to provide lobectomy with MLD, regardless of age. We have offered lobectomy to these patients based on meticulous assessments of cardiopulmonary performance, rather than based on age alone. However, the final decisions to select patients for surgery and the choice of surgical procedure may have been based on the judgements of the individual primary surgeons. Furthermore, heterogeneity of the study cohort may limit the ability to directly translate this study results to clinical practice. Especially, there was a higher rate of clinical stage IIIA patients in group 2 than in group 1 (P=0.016). For this reason, there was possibility for patients in Group 2 to be poor prognosis. The original point of this study was to analyze a dataset in which the surgical procedure was limited to lobectomy. Other, previous studies of surgical procedures have included partial resection and segmentectomy in their analyses, in addition to lobectomy (2,5,7,8,15-17). Accordingly, it would be difficult to obtain specific estimates of post-lobectomy survival, morbidity, and mortality outcomes from the results of these previous studies.
Conclusions
In this study of elderly patients with lung cancer, we found that maintenance of PS after lobectomy was associated with a good prognosis, while reductions in PS after lobectomy were associated with an extremely poor prognosis. History of cardiac ischemia and squamous cell carcinoma are risk factors post-lobectomy reductions in PS. Accordingly, careful patient evaluations and selection are needed when deciding whether to perform lobectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional review board approval was obtained for this study (ID: 29-011) and we waived the requirement for patient consent because of the retrospective nature of this study.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Takamochi K, Oh S, Matsuoka J, et al. Risk factors for morbidity after pulmonary resection for lung cancer in younger and elderly patients. Interact Cardiovasc Thorac Surg 2011;12:739-43. [Crossref] [PubMed]
- Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237-45. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. A model for morbidity after lung resection in octogenarians. Eur J Cardiothorac Surg 2011;39:989-94. [Crossref] [PubMed]
- Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg 2009;88:1093-9. [Crossref] [PubMed]
- Ambrogi V, Pompeo E, Elia S, et al. The impact of cardiovascular comorbidity on the outcome of surgery for stage I and II non-small-cell lung cancer. Eur J Cardiothorac Surg 2003;23:811-7. [Crossref] [PubMed]
- Miura N, Kohno M, Ito K, et al. Lung cancer surgery in patients aged 80 years or older: an analysis of risk factors, morbidity, and mortality. Gen Thorac Cardiovasc Surg 2015;63:401-5. [Crossref] [PubMed]
- Port JL, Kent M, Korst RJ, et al. Surgical resection for lung cancer in the octogenarian. Chest. 2004;126:733-8. [Crossref] [PubMed]
- Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. [Crossref] [PubMed]
- Murakami T, Nakamura Y, Hara M, et al. The impact of Functional Independence Measure score on the mortality of hemodialysis patients. Nihon Toseki Igakkai Zasshi 2014;47:129-36. [Crossref]
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009;301:513-21. [Crossref] [PubMed]
- Burfeind WR Jr, Tong BC, O'Branski E, et al. Quality of life outcomes are equivalent after lobectomy in the elderly. J Thorac Cardiovasc Surg 2008;136:597-604. [Crossref] [PubMed]
- Handy JR Jr, Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 2002;122:21-30. [Crossref] [PubMed]
- Takenaka T, Katsura M, Shikada Y, et al. The impact of cardiovascular comorbidities on the outcome of surgery for non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2013;16:270-4. [Crossref] [PubMed]
- Myrdal G, Gustafsson G, Lambe M, et al. Outcome after lung cancer surgery. Factors predicting early mortality and major morbidity. Eur J Cardiothorac Surg 2001;20:694-9. [Crossref] [PubMed]
- Mishra PK, Pandey R, Shackcloth MJ, et al. Cardiac comorbidity is not a risk factor for mortality and morbidity following surgery for primary non-small cell lung cancer. Eur J Cardiothorac Surg 2009;35:439-43. [Crossref] [PubMed]