Esophageal cancer developed in a radiated field: can we reduce the risk of a poor prognosis cancer?
Treatment options for patients with clinically localized cancer diagnosis include radiation therapy and chemotherapy. Each option is associated with side effects. Progress in cancer therapy has increased multimodality approach such as chemoradiotherapy, and patient’s life expectancy as well. However aggressive treatments and prolonged survival may cause growing concern about late effect of treatments. An important complication for patients submitted to combined approaches is the potential development of a second primary cancer (SPC). The appearance of SPCs is considered a late radiation effect only if it fits certain predetermined criteria. Some of these criteria include the timing of SPCs development (>5 years after radiation), the origin from tissues within the irradiated fields and the different histopathological features compared to primary tumors (1,2).
The process of radio-carcinogenesis is not clearly understood, and accurate risk models do not exist; the best data for radiation-induced carcinogenic risk come from A-bomb survivor studies, although these are subject to big limitations (3). Carcinogenesis risk after radiotherapy seems to be highest for tissues receiving even low doses (≤6 Gy) (4). However, there seems to be a tissues-specific dose-response relationship for radiocarcinogenesis, whit radiation-induced sarcomas developing in tissues receiving higher doses (30 to 60 Gy) and carcinomas induced in tissues receiving much lower doses (5,6). Both the integral dose to normal tissue and its dose distribution therefore influence the risk. Recently it has been demonstrated that multiple primary tumors arise from a single clone in several patients (7). Moreover, several evidences support the hypothesis that chemotherapy (essentially platinum-based) would either increase SPC incidence through damage to mucosal cells or decrease it by eliminating the potentially vulnerable cells. This compound acts mainly through the creation of intra-strand cross-links in the DNA, and cisplatin itself is considered to be an effective mutagen and carcinogen in vitro (8).
New techniques, as intensity-modulated radiotherapy (IMRT) seem to be superior to conformal three-dimensional conformal radiotherapy (3DCRT) in terms of coverage, conformity, and sparing of normal tissues. In addition, IMRT is superior in terms of functional sparing of critical organs at risk (OAR) and offers control and survival outcomes equivalent to those with 3DCRT. Concerns has been raised regarding potential carcinogenesis (9,10). When we consider IMRT as a replacement for conventional radiation treatment, two factors must be considered: (I) more monitor units (MU) are used, which results in a larger total-body radiation dose. Delivering a specified dose from a modulated field delivered by IMRT would require the accelerator to be energized for a longer time, and, hence, more MU will be needed; and (II) more fields are used, which results in a larger volume of normal tissue exposed to lower doses (11).
Relationship between smoking and esophageal tumors has been show. Data on smoking patients are limited by many factors. This kind of patients with history of cigarette smoking had a significant increase radiation-induced risk of second esophageal cancer with approximately estimated excess rate per Gy of 17% (12). The higher risks for squamous cell carcinomas (SCCs) reported in studies without individual dose estimates may reflect higher radiation doses from breast cancer radiotherapy to the upper and middle esophagus, where SCCs tend to occur, compared with the lower esophagus, site of prevalent adenocarcinomas development (13).
The risk of SPC occurrence is higher when radiotherapy is administrated at young age and children are considered to be ten times more sensitive to SPC radiation induced than adults are; similarly female gender shows an increased incidence compared to male (14).
The National Council on Radiation Protection and Measurements (NCRP) report 116, shows the relative probabilities of SPC developing by organ site (Table 1).
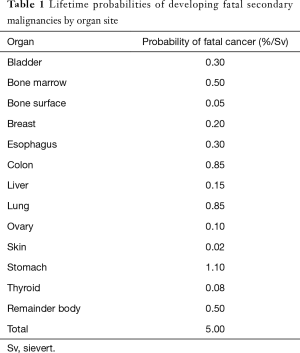
Full table
Lung and digestive tract carcinomas and sarcomas are the SPCs that more frequently occur after radiotherapy treatments, while stomach, colon and esophageal cancer are the most frequent among digestive tract cancer. Esophageal cancer incidence increases mostly in patients irradiated for Hodgkin and non-Hodgkin lymphomas, breast cancer, lung cancer and previous esophageal cancer (14,15).
Esophageal cancer is the eighth most common cancer and the sixth cause of cancer death worldwide (16). SCC and adenocarcinoma are the predominant histological types with the latter incidence increasing in western countries, while SCC remains very common in East areas. Despite treatment advances overall survival for this cancer remains poor, with a 5-year survival rate of 15–34% (17).
Surgical approach remains a milestone of esophageal cancer treatment in resectable disease. Nevertheless, several randomized clinical trials (RCTs) and meta-analysis showed that neo-adjuvant chemoradiotherapy or chemotherapy before surgical resection in locally advanced tumors can improve the prognosis of these patients without affecting postoperative morbidity and perioperative mortality rate (17-19).
The CROSS study (20) compared preoperative carboplatin and paclitaxel concurrent to radiotherapy followed by surgery and surgery alone, showing that neo-adjuvant therapy improves overall survival (49.4 vs. 20 months) and R0 resection (92% vs. 69%) with pathologic complete response rate of 29%. No difference was found in postoperative complication rate between the two groups (20,21).
As a consequence, neoadjuvant chemoradiotherapy (nCRT) followed by a radical surgery resection is actually the gold standard for locally advanced esophageal cancer accordingly to the CROSS study, recent meta-analysis (19) and guidelines (22).
However some issues remain unsolved: (I) preoperative chemoradiotherapy represents the best treatment option beyond histologic type? (18,19) (II) Should patients be selected concerning disease extension and nutritional status before to receive the trimodality approach? (III) The benefit and the treatment related complication showed in CROSS study may be translated into a wider population?
Most RCTs addressed the esophageal cancer neoadjuvant treatment pooling in the same study SCC and adenocarcinoma (20,21,23) while only few studies included a single histology; however, due to the poor cohort of patients enrolled, these studies are statistically underpowered. In order to answer the first question two recent meta-analysis performed subgroup analysis by histological type (18,19). Data obtained shows that concurrent nCRT followed by surgery improves outcome in SCC patients while this effect is much less evident in adenocarcinoma supporting the concept that esophageal SCC and adenocarcinoma are two different entities. Moreover several trials and a retrospective study suggest that there is a non-significant survival difference between neoadjuvant chemotherapy and nCRT in esophageal adenocarcinoma (24), aiming the question if the former should be the standard of care in this setting.
The second and the third questions deal with the external validity of RCTs. Randomized studies usually enroll a highly selected population of patients, often quite different from the real life model. When translated into clinical practice the results of treatments might be worse than expected. A RCT addressed the question whether patients with more extended disease (tumor length >8 cm, extension 2–4 cm into the gastric cardia or celiac node involvement), older age (<75 years) or worst nutritional status (>10% body weight loss) respect to patient eligible for CROSS study, had the same results of patients selected according CROSS study, when treated with nCRT (25). This study did not demonstrate difference in toxicity rate between the two groups; however, these patients characteristics had a prognostic value, with significant lower overall survival and disease free survival in the former group, and no difference from patient treated with definitive CRT, suggesting that selection of patients based on stage, age and nutritional status should be considered in the decisional algorithm of esophageal cancer treatment.
Esophageal cancer can arise in a previously irradiated field after treatment of another cancer or a previous esophageal cancer with a congruous interval of time. However, little is known about the optimal management and the prognosis of these secondary cancers.
Markar et al. performed a retrospective analysis of 2,489 consecutive patients undergoing surgical resection for esophageal cancer in Europe between 2000 and 2010 and among them identified a radiotherapy field esophageal cancer (ECRF) group comprising 136 patients. This group had a median latency delay between the primary cancer and esophageal cancer of 10 years.
Compared with the group of primary esophageal cancer a statistically significant difference in gender, with prevalent female (83.8% vs. 49.3%), upper third tumor location (48.5% vs. 11.9%), and SCC histology (86.0% vs. 42.7%) was shown. A less incidence of pathological stage III was found but R1/2 margins were more frequent in ECRF (21.3% vs. 10.9%) with the vertical margin mostly involved. Postoperative morbidity and 90 days mortality were significantly higher in previously irradiated patients, with longer hospital staying; however, this difference was not confirmed after adjustment on propensity scored aimed to reduce the effect of potential confounding factors (surgery after 2006, age ≥60 years, male incidence, ASA score, malnutrition, high center volume ≥80, TNM stage, tumor location, neoadjuvant chemotherapy, surgical technique and histology).
Treatment approach between ECRF and primary esophageal cancer was substantially different. Neoadjuvant treatment was rarely delivered in ECRF: 0% vs. 29.5% nCRT was recorded while neoadjuvant chemotherapy was administrated to 19% of ECRF patients vs. 47% of primary esophageal cancer.
The ECRF group reported here appears as a poor prognosis group with five years overall survival significantly reduced, compared to primary esophageal cancer (28.8% vs. 50.5%). The same reduction was seen in event free survival and cancer specific survival, while no difference was seen regarding to incidence and pattern of recurrence.
The authors argue that the increased incidence in R1 and R2 resection margins in ECRF, mostly related to vertical margin involvement, may be secondary to a multifocality of disease typical of irradiated fields. However the decreased overall and event free survival may also be explained by the substantial difference in treatment approach between the two groups; the ECRF group, mostly composed by SCC, did not receive nCRT, actually considered the gold standard in the treatment of esophageal SCC; this important limitation in ECRF patients treatment rather than biologically characteristics of higher aggressiveness of the disease, may account for the poorer prognosis. Authors conclude that the outcome in these patients appear to be influenced mostly by the limitation related to previous radiotherapy administration than to radiation induced carcinogenesis.
This retrospective study confirmed that female gender is predisposed to esophageal SPC than male and that SCC is the prevalent histology although adenocarcinoma account for 16.2% of cases. This cancer demonstrated a poor prognosis: due to high R1-R2 margins after surgery resection but also due to the impossibility to retreat previously irradiated patients with neoadjuvant radiotherapy in the same field. Only a small percentage of cases, presenting adenocarcinoma histology may benefit from neoadjuvant chemotherapy. As we know from CROSS study and recent meta-analysis, nCRT reduces R1-R2 margins and increases pathologic complete response, finally improving local disease control rate and overall survival in SCC esophageal cancer.
Considering the worse prognosis of esophageal, radiation induced SPC, the issue of sparing critic organs during the treatment for Hodgkin lymphoma, and, the breast and lung cancer appears to be of great relevance in the modern radiotherapy and, the reduction of esophageal SPC incidence will surely be one of the most important challenges for radiotherapy in the next future.
Several research areas may be feasible for the future:
- Patients selections: patients with defection in DNA repair or in the apoptotic process are probably more predisposed to secondary cancer. Patients with short telomeres are associated with susceptibility to carcinogenesis. However, the actually available data is not conclusive, and further research is required.
- Changes in radiotherapy: whenever side effects on the normal tissue are considered, it is advisable to irradiate a large target volume as long as this irradiation is not causing early or late side effects. In fact, the cost/benefit ratio is changing. The present data regarding the esophageal SPC demonstrated that the techniques should evolve from a maximal tolerable dose to a minimal effective radiation therapy. Large target volume irradiation with a moderate dose is likely to be associated with an increased risk.
- Dose reduction: whether it is confirmed that tissues exposed to a dose per fraction of less than about 120–150 mGy are associated with a low incidence of secondary cancer per dose unit, conformational and IMRT should be able to reduce this risk. It is known that esophageal SCC incidence decreased since the past two decades. This is probably related to the efforts, which have been made in, toward reduction of the doses delivered to the heart and mediastinal structures as esophagus, during the treatment of breast cancer, lung cancer or lymphoma.
- Dosimetric reasons: the estimation of the dose received by the various normal tissues is a difficult and time-consuming task, mostly regarding to the doses delivered to the tissues located at large distances from the edge of the treatment field, which is due to scatter of walls, ceiling, and neutron contribution.
In conclusion, even if current practice substantially changed radiation doses to organs, the general message remains unchanged: the secondary esophageal cancer risk from radiotherapy in adulthood is relatively small, especially when compared with the treatment benefit; however, the prognosis of these patients remains poor and any effort to minimize the risk is needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Amemiya K, Shibuy H, Yoshimura R, et al. The risk of radiation-induced cancer in patients with squamous cell carcinoma od the head and keck and its results of treatment. Br J Radiol 2005;78:1028-33. [Crossref] [PubMed]
- Cahan WG, Woodward HQ, Higinbotham NL, et al. Sarcoma arising in irradiated bone. Cancer 1948;1:3-29. [Crossref] [PubMed]
- International Commission of Radiological Protection. Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP 1990;1991:21.
- Dörr W, Herrmann T. Second primary tumors after radiotherapy for malignancies. Treatment-related parameters. Strahlenther Onkol 2002;178:357-62. [Crossref] [PubMed]
- Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003;56:83-8. [Crossref] [PubMed]
- Murray EM, Werner D, Greeff EA, et al. Postradiation sarcomas: 20 cases and a literature review. Int J Radiat Oncol Biol Phys 1999;45:951-61. [Crossref] [PubMed]
- Bedi GC, Westra WH, Gabrielson E, et al. Multiple head and neck tumors: evidence for a common clonal origin. Cancer Res 1996;56:2484-7. [PubMed]
- Greene MH. Is cisplatin a human carcinogen? J Natl Cancer Inst 1992;84:306-12. [Crossref] [PubMed]
- Hall EJ, Phil D. Intensity-modulated radiation therapy, protons and the risk of second cancers. Int J Radiat Oncol Biol Phys 2006;65:1-7. [Crossref] [PubMed]
- Nutting CM, Convery DJ, Cosgrove VP, et al. Improvements in target coverage and reduced spinal cord irradiation using intensity-modulated radiotherapy (IMRT) in patients with carcinoma of the thyroid gland. Radiother Oncol 2001;60:173-80. [Crossref] [PubMed]
- Morton LM, Gilbert ES, Hall P, et al. Risk of treatment-related esophageal cancer among breast cancer survivors. Ann Oncol 2012;23:3081-91. [Crossref] [PubMed]
- Thilmann C, Sroka-Perez G, Krempien R, et al. Inversely planned intensity modulated radiotherapy of the breast including the internal mammary chain: A plan comparison study. Technol Cancer Res Treat 2004;3:69-75. [Crossref] [PubMed]
- Ahsan H, Neugut AI. Radiation therapy for breast cancer and increased risk for esophageal carcinoma. Ann Intern Med 1998;128:114-7. [Crossref] [PubMed]
- Kumar S. Second malignant neoplasms following radiotherapy. Int J Environ Res Public Health 2012;9:4744-59. [Crossref] [PubMed]
- Nation Council on radiation Protection and Measurement. Report No. 116: Limitation of exposure to ionizing radiations. Bethesda, MD: NCRP, 1993. Available online: https://www.ncrppublications.org/Reports/116
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Deng HY, Wang WP, Wang YC, et al. Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur J Cardiothorac Surg 2017;51:421-31. [PubMed]
- Liu B, Bo Y, Wang K, et al. Concurrent neoadjuvant chemoradiotherapy could improve survival outcomes for patients with esophageal cancer: a meta-analysis based on random clinical trials. Oncotarget 2017;8:20410-7. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- de Heer EC, Hulshoff JB, Klerk D, et al. Effect of Extending the Original Eligibility Criteria for the CROSS Neoadjuvant Chemoradiotherapy on Toxicity and Survival in Esophageal Cancer. Ann Surg Oncol 2017;24:1811-20. [Crossref] [PubMed]
- Markar SR, Bodnar A, Rosales J, et al. The impact of neoadjuvant chemoradiotherapy on perioperative outcomes, tumor pathology, and survival in clinical stage II and III esophageal cancer. Ann Surg Oncol 2013;20:3935-41. [Crossref] [PubMed]


