Development of a novel ex vivo porcine laparoscopic Heller myotomy and Nissen fundoplication training model (Toronto lap-Nissen simulator)
Introduction
Laparoscopic surgery has become a major component of general surgery. Studies have shown that laparoscopic fundoplication has multiple benefits over the traditional open approach, including improved cosmesis, reduced morbidity, decreased hospital stay, decreased respiratory complications, and faster recovery (1-3). The long-term outcomes of laparoscopic fundoplication are comparable to open surgery in subjective symptoms. (4-6). Laparoscopic surgery has become the preferred surgical option (7,8). However, some reports have described higher risks for complications with the laparoscopic approach, such as wrap migration, esophageal perforation, acute para-esophageal herniation, and stenosis of the esophageal hiatus and pneumothorax (9-11).
Studies have shown a higher reoperation rate at the early learning stages of the laparoscopic approach, which diminishes with increasing experience (9,12,13). Before the advent of the 80-hour workweek policy, trainees in general surgery reported inadequate exposure to advanced laparoscopic cases (14). Reduced time in the hospital may lead to further failure in advanced laparoscopic training. A single institution retrospective case review shows an increased rate of conversion to laparotomy in the first laparoscopic repair 30 cases, and an increased operative time and hospital stay in the first 26 cases (15). Meta-analysis of eleven studies (1,558 patients) evaluating operative time, conversion rate, complication rate, and hospital stay revealed a mean of 28 cases to achieve proficiency in laparoscopic fundoplication (16). The attainment of skills in laparoscopic surgery, and the need to acquire capability of using new instruments, suturing techniques, and new technological devices, are challenges in performing a safe and effective operation (7,9).
A study from a community program suggests that residents can be trained for proficiency in the operating room with an adequate number of cases in a small training program (17). Currently, animal tissue models are used regularly at skill centers to train for laparoscopic procedures, such as myotomy and fundoplication outside the clinical setting (18-20). However, there have been few models that replicate the anatomical orientations of organs as found in the live animal setting (20). We developed an arch system that produces an anatomically relevant model of the upper abdomen for teaching laparoscopic myotomy and fundoplication, termed the “Toronto lap-Nissen simulator”. This simulator can be combined with instruction and step-by-step demonstration videos to bridge the early learning curve in a safe environment outside the clinical setting. Our clinically relevant simulator will simplify the trainees’ transition for performing laparoscopic foregut procedures.
Methods
We integrated the use of an inexpensive ex vivo porcine training model as a part of our laparoscopic skills laboratory-training curriculum. Afterwards, we surveyed trainees to evaluate the observed benefit of the learning session.
Materials
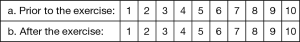
The Toronto lap-Nissen simulator was developed by the authors and medical engineers (M.B. and G.A.) at the Toronto General Hospital (Figure 1A). The prototype was made in-house with an acrylic plate and iron mesh. The two arch systems cost $300 CAD each.
Intact porcine organs including the lung, heart, esophagus, stomach, spleen, and liver were purchased from a local meat butcher at a cost of $70 CAD per organ bloc. The butcher transported the porcine organs one to two days before a training session. The porcine organs were kept in cold temperature with ice blocks during transit. After receiving the organs at our institution, these organs were kept in a refrigerator room at −4 °C. We removed the lung, heart and liver from the organ bloc for separate use in thoracoscopic training. The estimated cost of the animal organs required for this study, including the esophagus, stomach, spleen and diaphragm, is less than $35 CAD per simulator.
The arch (Figure 1A) was constructed with 5 clips attached to the half dome part and with 6 clips in the bottom part. The simulator (Figure 1B) included the stomach, with the omentum minor and major attached by the short gastric vessels (vasa brevia) and the esophagus, which entered the mediastinum through the opening between the crus. The esophagus was exposed by dissecting the peritoneal layer covering the crus. The simulator also included the spleen to which the short gastric vessels were connected. The diaphragm was suspended by an arch inside of a training torso, fitted with trocar ports for the insertion of instruments (Figure 1C). In the setup, the clips held the diaphragm of the organ bloc to create adequate tension akin to a chest cavity. An 8Fr intubation tube was inserted into the esophagus which was fixed to the cranial side of the model, creating adequate tension on the esophagus, in order to better mimic the elasticity of human esophagus. Standard laparoscopic equipment was utilized, including a camera with a 10-mm 0°-telescope, a high definition 19-inch monitor, and a light source.
Study population and design
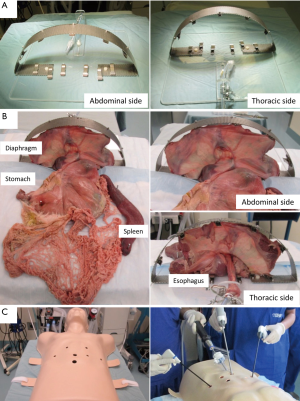
The study was completed by the trainees (n=25) and faculty members (n=5) of the Toronto Interventional Thoracic Surgery Training Course at the MaRS Discovery District from July 15th to 18th, 2015 and from July 13th to 16th 2016. The training session began with the distribution of written materials and a didactic session on laparoscopic Heller myotomy and Nissen fundoplication, including topics on anatomy, operative procedure, and complications. This was followed by faculty using the developed training model to demonstrate the techniques used in laparoscopic myotomy and fundoplication on the simulator. Emphasis was placed on the fundamental aspects of advanced laparoscopic surgery such as the use of both intra- and extracorporeal knot tying and the use of the energy devices. Additionally, teaching emphasis was placed on the dissection and ligation of the short gastric vessels, mobilization of the fundus, the subsequent creation of a retro esophageal space, and the creation of a loose 360-degree fundoplication. After the demonstration, trainees were given the opportunity to attempt laparoscopic myotomy and fundoplication on a fresh set of organs under one-on-one guidance by the faculty (Figure 2).
Survey technique
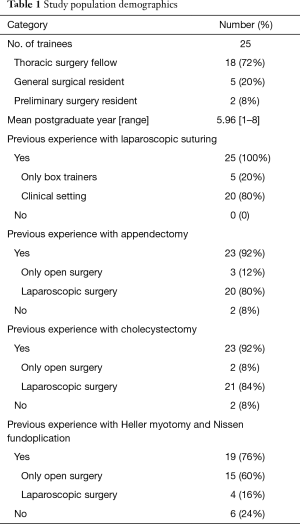
At the end of the training session for the laparoscopic Heller myotomy and Nissen fundoplication, the participants were asked to fill out a survey to evaluate the practicality of using the simulator as a training tool (Table 1). Trainees filled out the survey anonymously. Survey demographic data included training level, categorical status, and prior surgical experience. Training session evaluation data included value of time spent in the laboratory, transferability of skills to the operating room, realism, difficulty, overall laparoscopic skill set value, and desire to repeat the exercise. Additionally, trainees were surveyed for both pre- and post-training knowledge level as well as comfort level in assisting and performing a laparoscopic myotomy and fundoplication (20).
Full table
Statistical methods
The trainees’ mean subjective ratings of pre- and post-training in (I) knowledge of the procedure; (II) comfort assisting with the procedure; and (III) comfort performing the procedure as the primary surgeon were treated as ordinal variables for all analyses. Means, standard deviations, and number of observations were recorded for each variable. The experimental unit was each individual subject (n=25). The experiment used a repeated measures design with each subject's response evaluated at two periods (pre- and post-training). The null hypothesis was that there would be no difference between two survey results. Differences between pre- and post-training ratings (rejection of the null hypothesis) were compared using two-tailed paired student’s t-tests with a 95% confidence interval. Statistical analyses were conducted using R (version 3.0.1; R Development Core Team). All P values were based on a two-sided hypothesis, and a P value of <0.05 was considered to have statistical significance.
Results
Study population demographics
Twenty-five trainees received training using the Toronto lap-Nissen simulator. All had previous experience with laparoscopic suturing, either in the clinical setting or with box trainers. Of the twenty-five trainees, nineteen (76%) had previous experience with Heller myotomy and Nissen fundoplication. Among this group, only 4 (16%) had experience with laparoscopic Heller myotomy and Nissen fundoplication.
The average number of years post-graduation was 5.96 years (range, 1 to 8). Eighteen (72%) trainees were categorical thoracic surgery fellows; five (20%) were categorical general surgical residents; and two (8%) were preliminary surgery residents (Table 1).
Survey data
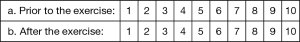
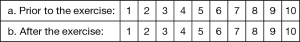
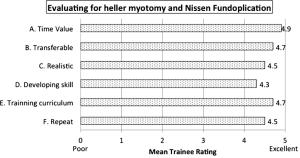
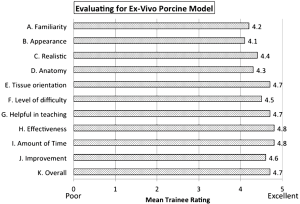
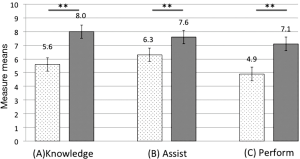
The evaluation of the laparoscopic Heller myotomy and Nissen fundoplication training exercise is described in Figure 3. Mean rating (scale 1–5) to each question as follows: (A) the laparoscopic Nissen Exercise was a valuable use of time: 4.9; (B) the skills I learned will transfer to the operating room: 4.7; (C) the exercise was realistic: 4.5; (D) this exercise helped develop my overall general laparoscopic skill set and will improve my ability to perform and assist in other laparoscopic procedures: 4.3; (E) this exercise should remain in the laparoscopic training curriculum: 4.7; and (F) repeating this exercise a second time in the future would be beneficial: 4.5, are all described in Figure 3. All trainees reported that participating in the exercise was a valuable use of time, that it was appropriately difficult, and that the skills acquired will transfer to the operating room. The evaluation of the ex vivo porcine training model is described in Figure 4. Mean rating (scale 1–5) to each question as follows: (A) familiarity with procedures: 4.2; (B) model appeared lifelike: 4.1; (C) tissue felt realistic: 4.4; (D) porcine anatomy similar to human: 4.3; (E) model orientation was accurate: 4.7; (F) appropriate level of difficulty: 4.5; (G) helpful in teaching procedures: 4.7; (H) evaluator was effective: 4.8; (I) appropriate amount of time: 4.8; (J) experience will translate into improvement in real procedures: 4.6; and (K) overall satisfaction is high: 4.7. Significant improvements in subjective measure means (scale 1–10) can be seen pre- vs. post-training (Figure 5): (A) knowledge level (5.6 vs. 8.0, P<0.001); (B) comfort assisting with a laparoscopic myotomy and fundoplication (6.3 vs. 7.6, P<0.001); and (C) comfort performing a laparoscopic myotomy and fundoplication as the primary surgeon (4.9 vs. 7.1, P<0.001).
Discussion
We developed an ex vivo porcine training model to teach surgical trainees the basics of performing laparoscopic Heller myotomy and Nissen fundoplication. According to the trainees in this study, the newly developed simulator is a valuable training tool for these laparoscopic procedures. It is less expensive than a live animal model and setup is simple for training. The use of our inexpensive ex vivo porcine training model increases surgical trainee comfort level in performing a laparoscopic fundoplication procedure in the operating room. In addition, trainees reported that it is a valuable educational tool that helped improve their overall laparoscopic skill set. Trainees also reported their desire to return to the laboratory and repeat the exercise, verifying that it is a valuable use of their time.
Training outside the clinical setting is important to reduce the incidence rate of esophageal perforations and pneumothorax in real human cases. Animal tissue models and live animals are used regularly to train for procedures before they are performed on patients (18,19). However, as articulated in the statements of the American College of Surgeons, only as many animals as necessary should be used. Any pain or distress that animals may experience should be minimized or alleviated, and wherever feasible, alternatives to the use of live animals should be developed and used. Furthermore, live animals are costly (20). The artificial organ model is a sufficient tool for laparoscopic training. However, the artificial model still needs improvement because the artificial stomach is too rigid to wrap the esophagus as performed in Nissen Fundoplication procedures (21,22). Furthermore, human and animal tissues are complex multilayered materials and are difficult to replicate artificially as their properties depend on the amount of layers, layer thickness, orientation of muscle fibers, moisture level, temperature, etc. For example, the stomach wall is composed of tunica mucosa, tela submucosa, tunica muscularis, tela subserosa, and tunica serosa from inner layer to outer layer (23). While preparing animal organs to replicate the exact mechanical properties of human abdominal tissue remains a challenge, an animal tissue training model is still ideal for learning techniques in dissecting tissue (e.g., tissue connected to the esophagus). Extracted animal organs from a butcher are also more cost effective than living animals. Another specific advantage of our simulator is the anatomically appropriate positioning of the diaphragm– an essential simulation for the fundoplication procedure.
Our simulator allows training and objective assessment of the essential technical skills for crural closure and creation of a wrap around the esophagus to minimize complications in the clinical setting. Trainees have the opportunity to acquire fundamental skills before attempting myotomy and fundoplication in the operating room. Additionally, they can refine their skills by focusing on areas of the procedure they perceive as weaknesses and achieving further independent technical proficiency. Overall, the trainees’ response to the ex vivo porcine training model has been significantly positive. The training laboratory provides a setting in which trainees can ask questions and receive feedback without the time and cost constraints of the operating room. Furthermore, errors such as gastrotomy or esophageal perforation do not carry the same consequence as they do in actual patients, allowing the training of more inexperienced first-year residents without risk of patient injury. The ease of storage and minimal cost of the organs provides ample opportunity for residents to repeat the exercise an unlimited number of times until they feel capable. The combination of all of these factors may lead to a faster and shallower learning curve in the operating room.
A limitation of this study is the reliance on subjective evaluation. Follow-up objective studies will be required to validate this initial experience. The definitive measure of the efficacy of this simulator is a randomized control study comparing fundoplication in the operating room performed by residents who received training with developed simulator vs. those that did not. Additionally, such a study could validate the hypothesis that the early portion of the learning curve can be shifted to the training laboratory setting. Supplementary investigation is required to ascertain the benefit of the laboratory for residents with prior laparoscopic fundoplication experience.
Conclusions
We developed an inexpensive and easily reproducible training simulator for laparoscopic procedures. This newly developed ex vivo porcine model with an arch system simulates human anatomy and increases trainees’ comfort level in performing and assisting with myotomy and fundoplication. We believe that this training simulator will become a significant addition to laparoscopic training.
Ex vivo Nissen fundoplication model survey sheet
- What post-graduated year will you complete in June 2016? Year___
- Are you a category General Surgery Resident? Yes or No
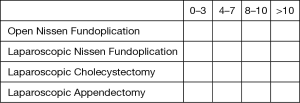
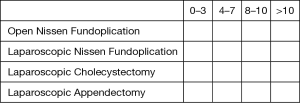
If not, please specify transitional year or subspecialty: ___ - Quantify your experience as a primary surgeon with respect to the following cases
- Quantify your experience as a first assistant with respect to the following cases
Evaluating for laparoscopic Nissen fundoplication)
Disagree strongly: 1; disagree slightly: 2; no opinion: 3; agree slightly: 4; agree strongly: 5
- The laparoscopic Nissen exercise was a valuable use of time: ___
- The skills I learned will transfer to the operating room: ___
- The exercise was realistic: ___
- This exercise helped develop my overall general laparoscopic skill set and will improve my ability to perform and assist in other laparoscopic procedures: ___
- This exercise should remain in the laparoscopic training curriculum: ___
- Repeating this exercise a second time in the future would be beneficial: ___
Evaluating for ex vivo porcine model
Disagree strongly: 1; disagree slightly: 2; no opinion: 3; agree slightly: 4; agree strongly: 5
- Familiarity with procedures: ___
- Model appeared lifelike: ___
- Tissue felt realistic: ___
- Porcine anatomy similar to human: ___
- Model orientation was accurate: ___
- Appropriate level of difficulty: ___
- Helpful in teaching procedures: ___
- Evaluator was effective: ___
- Appropriate amount of time: ___
- Experience will translate into Improvement in real procedures: ___
- Overall satisfaction is high:___
Comparing “prior to the exercise” with “after the exercise”: scale from 1 (low) to 10 (high)
- My knowledge of the laparoscopic Nissen fundoplication was
- My comfort level in assisting with laparoscopic Nissen Fundoplication
- My comfort level in performing a Laparoscopic Nissen as the primary surgeon
(Any comments)_____________________________________________________
Thank you so much for your survey…
Acknowledgements
We would like to thank Mr. Maciej Bauer and Mr. Gad Acosta for development of the simulator and production of the prototypes, as well as Ms. Alexandria Grindlay, Ms. Judy McConnell and Ms. Kimberley Hudson for supporting the research work. H Ujiie received a research scholarship from the Joseph M. West Family Memorial Fund.
Footnote
Conflicts of Interest: This study was presented on the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) 2016 Annual Meeting, Boston, MA, USA 16th–19th March, 2016.
References
- Nilsson G, Larsson S, Johnsson F. Randomized clinical trial of laparoscopic versus open fundoplication: blind evaluation of recovery and discharge period. Br J Surg 2000;87:873-8. [Crossref] [PubMed]
- Catarci M, Gentileschi P, Papi C, et al. Evidence-based appraisal of antireflux fundoplication. Ann Surg 2004;239:325-37. [Crossref] [PubMed]
- Peters MJ, Mukhtar A, Yunus RM, et al. Meta-analysis of randomized clinical trials comparing open and laparoscopic anti-reflux surgery. Am J Gastroenterol 2009;104:1548-61; quiz 7, 62.
- Draaisma WA, Rijnhart-de Jong HG, Broeders IA, et al. Five-year subjective and objective results of laparoscopic and conventional Nissen fundoplication: a randomized trial. Ann Surg 2006;244:34-41. [Crossref] [PubMed]
- Nilsson G, Wenner J, Larsson S, et al. Randomized clinical trial of laparoscopic versus open fundoplication for gastro-oesophageal reflux. Br J Surg 2004;91:552-9. [Crossref] [PubMed]
- Salminen P, Hurme S, Ovaska J. Fifteen-year outcome of laparoscopic and open Nissen fundoplication: a randomized clinical trial. Ann Thorac Surg 2012;93:228-33. [Crossref] [PubMed]
- Soot SJ, Eshraghi N, Farahmand M, et al. Transition from open to laparoscopic fundoplication: the learning curve. Arch Surg 1999;134:278-81; discussion 82. [Crossref] [PubMed]
- Bais JE, Bartelsman JF, Bonjer HJ, et al. Laparoscopic or conventional Nissen fundoplication for gastro-oesophageal reflux disease: randomised clinical trial. The Netherlands Antireflux Surgery Study Group. Lancet 2000;355:170-4. [Crossref] [PubMed]
- Watson DI, Baigrie RJ, Jamieson GG. A learning curve for laparoscopic fundoplication. Definable, avoidable, or a waste of time? Ann Surg 1996;224:198-203. [Crossref] [PubMed]
- Lafullarde T, Watson DI, Jamieson GG, et al. Laparoscopic Nissen fundoplication: five-year results and beyond. Arch Surg 2001;136:180-4. [Crossref] [PubMed]
- Hunter JG, Smith CD, Branum GD, et al. Laparoscopic fundoplication failures: patterns of failure and response to fundoplication revision. Ann Surg 1999;230:595-604; discussion -6.
- Zacharoulis D, O'Boyle CJ, Sedman PC, et al. Laparoscopic fundoplication: a 10-year learning curve. Surg Endosc 2006;20:1662-70. [Crossref] [PubMed]
- Gill J, Booth MI, Stratford J, et al. The extended learning curve for laparoscopic fundoplication: a cohort analysis of 400 consecutive cases. J Gastrointest Surg 2007;11:487-92. [Crossref] [PubMed]
- Rattner DW, Apelgren KN, Eubanks WS. The need for training opportunities in advanced laparoscopic surgery. Surg Endosc 2001;15:1066-70. [Crossref] [PubMed]
- Deschamps C, Allen MS, Trastek VF, et al. Early experience and learning curve associated with laparoscopic Nissen fundoplication. J Thorac Cardiovasc Surg 1998;115:281-4; discussion 4-5. [Crossref] [PubMed]
- Dagash H, Chowdhury M, Pierro A. When can I be proficient in laparoscopic surgery? A systematic review of the evidence. J Pediatr Surg 2003;38:720-4. [Crossref] [PubMed]
- Reynolds FD, Goudas L, Zuckerman RS, et al. A rural, community-based program can train surgical residents in advanced laparoscopy. J Am Coll Surg 2003;197:620-3. [Crossref] [PubMed]
- Aggarwal R, Boza C, Hance J, et al. Skills acquisition for laparoscopic gastric bypass in the training laboratory: an innovative approach. Obes Surg 2007;17:19-27. [Crossref] [PubMed]
- van Velthoven RF, Hoffmann P. Methods for laparoscopic training using animal models. Curr Urol Rep 2006;7:114-9. [Crossref] [PubMed]
- Jensen AR, Milner R, Gaughan J, et al. An inexpensive ex-vivo porcine laparoscopic Nissen fundoplication training model. JSLS 2005;9:322-7. [PubMed]
- Botden SM, Goossens R, Jakimowicz JJ. Developing a realistic model for the training of the laparoscopic Nissen fundoplication. Simul Healthc 2010;5:173-8. [Crossref] [PubMed]
- Botden SM, Christie L, Goossens R, et al. Training for laparoscopic Nissen fundoplication with a newly designed model: a replacement for animal tissue models? Surg Endosc 2010;24:3134-40. [Crossref] [PubMed]
- Netter FH. editor. Atlas of Human Anatomy. 6th ed. New York: Elsevier Health Sciences, 2014.