A review of clinical practice guidelines for lung cancer
Introduction
A challenge for health professionals managing patients with lung cancer is to keep abreast with the rapidly growing evidence base in diagnosis, staging and treatment. Clinical practice guidelines for lung cancer provide a useful tool of synthesised evidence to guide complex clinical decision making. They have the potential to enhance the healthcare decisions of clinicians and patients, and to lead to better quality care and improved outcomes for patients when they are of high quality, accessible and successfully implemented (1).
Numerous guidelines have been developed for lung cancer across the world. With a vast number of lung cancer guidelines developed in different countries by different organisations and listed across numerous guideline databases, this review article aims to provide a comprehensive overview of available guidelines for lung cancer available in English language. Key features such as developing organisation(s), publication date, geographic context and access details are listed for each guideline. More detailed information in regards to the methodology, the dissemination and implementation approach, important background information and any associated resources are briefly summarised in the results section.
Methods
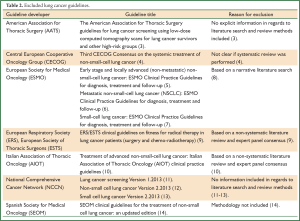
Clinical practice guidelines are defined as “statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options” (1). This definition has been used to identify clinical practice guidelines for lung cancer to be included in this review article. A comprehensive literature search consisting of searching the Guidelines International Network (GIN) International Guideline Library, National Guideline Clearinghouse, Standards and Guidelines Evidence (SAGE) portal, Australia’s Clinical Practice Guideline Portal, PubMed as well as Scottish International Guidelines Network’s (SIGN) and National Institute for Health and Care Excellence’s (NICE) databases was completed. In addition, snowballing was used to identify any further relevant guidelines that were missed in the database searches. The results were then screened and included if the following criteria were met (Table 1).

Full Table
Guidelines addressing malignant pleural mesothelioma, thymoma, specific symptom management topics and other secondary topics were out of scope for this review article and therefore not considered. Clinical practice guidelines that met all criteria, but were based on a non-systematic literature review, were excluded from this review as the systematic review requirement according to the clinical practice guideline definition was not met (Table 2). Other forms of clinical guidance such as general consensus statements on clinical topics, expert advice, task force reports, health technology appraisal and appropriate use criteria were also excluded.
Full Table
Results
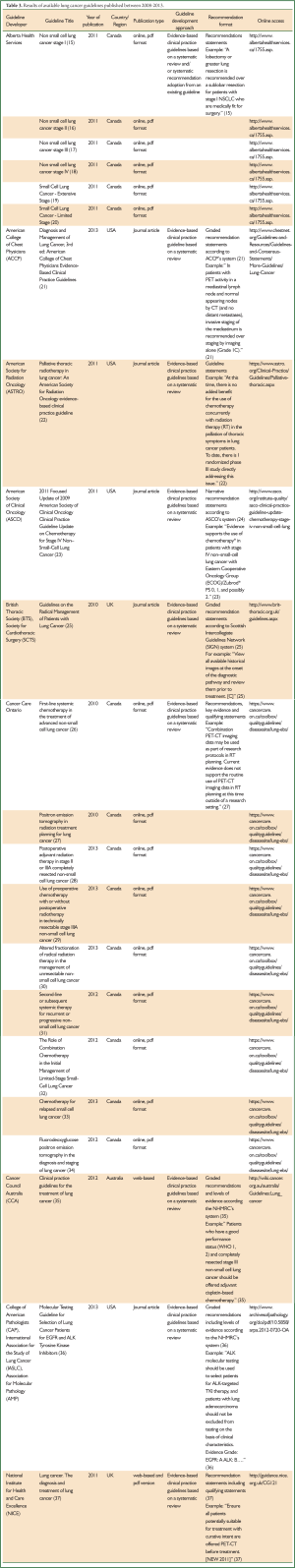
In total 22 lung cancer guideline documents developed by 12 different organisations were identified as meeting the inclusion criteria (Table 3).
Full Table
Table 3 shows that there is wide variation in nearly every aspect of guideline development between each of the guidelines. As Table 3 indicates, the scope varied across the identified guidelines. Some guideline developers, such as NICE (37) or American College of Chest Physicians (ACCP) (21), published their lung cancer guideline as one large document covering all areas of lung cancer from epidemiology, screening, diagnosis, treatment, follow-up to end-of-life care. Others, such as Alberta Health Services (15-20) and College of American Pathologists (CAP), International Association for the Study of Lung Cancer (IASLC) and Association for Molecular Pathology (AMP) (36), developed more focused guidelines addressing specific area(s) of lung cancer. For example, Alberta Health Services released their lung cancer guidelines as separate discrete publications and published a guideline for each stage (15-20). Cancer Care Ontario’s guidelines are even more specific and address only one or a few closely related clinical questions (26-34).
The guideline development approach also varied between organisations. All included clinical practice guidelines are based on formal systematic reviews to generate evidence-based recommendations. However, the evidence assessment tools, recommendation format and recommendation grading schemes vary (Table 3). A few developers even have a procedure in place to evaluate if recommendations from existing guidelines could be formally adopted (38) as opposed to developing de novo guidelines/recommendations.
Whereas all identified guidelines are disseminated and accessible online, the presentation varied from documents available for download, web-based clinical practice guidelines, guidelines available as published journal articles or a combination of approaches (Table 3). Many developers offer printed guideline copies or printed summaries of the recommendations available upon request.
The subsequent section summarises background information in regards to the relevant guideline(s), composition of guideline development group, conflict of interest (COI) management, guideline funding, the methodological as well as dissemination and implementation approach, planned update and any associated resources for the guideline(s) in narrative form under each guideline developer or collaboration of guideline developers. Together with Table 3, the summarised information covers the subject areas of the standards identified by the Institute of Medicine for developing trustworthy clinical practice guidelines (establishing transparency, management of COI, guideline development group composition, systematic review, evidence foundations and evidence level ratings, recommendation formulation, external review and updating) (1). Dissemination and implementation approach and any associated guideline resources were added for each guideline as these are key to achieve successful guideline uptake (39).
Alberta Health Services
Introduction
Alberta Health Services is a Canadian health authority that delivers health services in the Canadian province Alberta and develops clinical practice guidelines in oncology. For each stage in non-small cell and small cell lung cancer a separate lung cancer guideline document was produced. They are published as separate PDF publications on Alberta Health Services’ website (Table 3) (15-20).
Guideline development methodology
Guideline development at Alberta Health Services follows a systematic guideline development approach as detailed in the Guideline Utilization Resource Unit Handbook (40). For each lung guideline, a multidisciplinary working group was recruited. The guideline scope was defined and clinical questions developed. The literature searches were carried out by an in-house knowledge management specialist. All retrieved literature results were screened, assessed and synthesised. Existing guidelines were also searched for in order to evaluate if an existing guideline could be formally adopted. Any retrieved existing guidelines were formally assessed with the AGREE II instrument to ensure minimum requirements for a good quality guideline were met, before considering the formal adoption or adaption of existing recommendation(s). Guideline recommendations were developed and formulated by the guideline working group members based on the evidence tables and expert clinical interpretation or, if applicable, an existing guideline. Recommendations were formulated in the form of action statements and the reasoning behind the recommendation, including the quality and level of evidence, was added in narrative form. Alberta Health Services did not use a formal grading scheme to assign specific grades to recommendations. The draft guidelines were then open for comment and reviewed by all members of the Provincial Tumour Team. Once the guideline documents were finalised, they were formally endorsed by Alberta Health Services.
COI management
COI statements are included in each lung cancer guideline as well as an overall statement from the developer that each guideline was satisfactorily developed in an unbiased manner (40).
Funding
Each lung cancer guideline document states that there was no direct industry involvement in the production or dissemination of the guideline (40).
Dissemination and implementation approach
The guidelines are published on the Alberta Health Services website. All members are notified when a guideline has been updated or added. Guidelines are presented at the local and provincial tumour team meeting as well as weekly hospital rounds to facilitate uptake (40).
Planned update
Alberta Health Services clinical practice guidelines are reviewed and updated every one to two years (40).
Associated resources
Treatment algorithms for each lung cancer guideline are available from http://www.albertahealthservices.ca/1755.asp.
American College of Chest Physicians
Introduction
The ACCP produces guidelines in chest medicine and has developed guidelines for lung cancer since 2003. The third edition of the ACCP lung cancer guidelines has been published in 2013 and is included in this review (Table 3) (21).
Guideline development methodology
ACCP used a formalised, systematic approach to develop the third edition of the lung cancer guidelines. A selected expert lung cancer guideline panel developed research questions in PICO [The acronym PICO refers to the 4 elements that should be included in a structured clinical question to govern systematic searches: patient, intervention, comparison and outcome. A framework commonly used in evidence-based medicine (41).] format and literature searches were designed and completed. The literature results were then screened against inclusion and exclusion criteria and formally assessed using standard quality assessment tools. If applicable, good quality meta-analysis (already published or performed by the authors specifically for the guideline) were used to inform the recommendations. Evidence summary tables and profiles were compiled for most PICO questions. Based on the evidence tables, recommendations were formulated and then graded according to the ACCP recommendation grading system. The whole guideline panel reviewed the guideline content, including formal anonymous voting to approve recommendations during face-to-face and virtual meetings. The draft guideline was then submitted through an internal and external review process before the guideline was finalised and published (42).
COI management
Each nominated guideline panel member had to submit a COI statement before the start of the guideline project. The COI statements were reviewed by the Guidelines Oversight Committee. All panellists were required to submit an updated COI statement before each meeting. COI management included strategies such as not drafting or voting on recommendations that were related to a particular conflict (42).
Funding
The majority of the guideline was funded by the ACCP. One private foundation and one pharmaceutical company financially supported the development and dissemination of the guideline. Those sponsoring companies were not allowed to participate in the guideline development process (42).
Dissemination and implementation approach
The ACCP lung guidelines are disseminated through the College’s website (www.chestnet.org), the CHEST journal publication, National Guidelines Clearinghouse and GIN Library (42).
Planned update
The start of ongoing review is planned 1 year after publication unless the content experts, who continue to monitor the literature, suggest that recommendations need to be updated (42).
Associated resources
Additional clinical resources will be accessible in Chest Evidence. Associated patient guides will be available from www.onebreath.org (42).
American Society for Radiation Oncology (ASTRO)
Introduction
The Guidelines Subcommittee of the ASTRO identified a need for an evidence-based guideline on the use of palliative radiotherapy to lung cancer patients. The project proposal to develop this guideline was submitted and approved by the ASTRO Board of Directors in 2009 (22).
Guideline development methodology
A task force was established and assigned to review and synthesize the current available evidence to develop this guideline. The task force was divided into three topic groups and a systematic literature review was completed for each area. Evidence assessment, including the creation of evidence tables, and the formulation of the guideline content were completed and then revised by the complete expert group. The final draft was then circulated to three expert reviewers, the ASTRO legal counsel and also published on the ASTRO website for public comment. The feedback was reviewed and incorporated before the guideline was finally reviewed and approved by the ASTRO Board of Directors (22).
COI management
At the beginning of the guideline project, all members submitted COI declarations. The task group chairs reviewed all COI statements and determined that the disclosures would have no impact upon the content of the guideline manuscript (22).
Funding
Details in regards to the funding of the guidelines were not specified in the guideline document.
Dissemination and implementation approach
The guideline was formally published in the journal Practical Radiation Oncology (22) and the link to the article is listed on ASTRO’s website.
Planned update
The ASTRO Guidelines Subcommittee will monitor this guideline and initiate an update when appropriate (22).
Associated resources
Not identified.
American Society for Clinical Oncology
Introduction
The American Society of Clinical Oncology (ASCO) has been developing clinical practice guidelines for lung cancer since 1997 and has published an update on chemotherapy for stage IV non-small lung cancer in 2011 that was eligible for inclusion in this review (23).
Guideline development methodology
The 2011 update on chemotherapy treatment for stage IV lung cancer is based on ASCO’s 2009 lung cancer guideline update and addressed the clinical question, “What is the optimal duration of first-line chemotherapy for stage IV NSCLC?” from the previous guideline. The literature search for this guideline included an update of the original 2009 literature search and a systematic assessment of the updated evidence. ASCO’s Guideline Procedures Manual provides details about ASCO’s methodology for guideline development (24). The 2011 focused update was drafted by the co-chairs of the 2009 guideline as well as ASCO staff and was then circulated to the entire update committee for approval. The final document was reviewed and approved by ASCO’s Clinical Practice Guideline Committee and Board of Directors Executive Committee. It was then submitted to Journal of Clinical Oncology for peer review before being finalized and published (23).
COI management
All members of the update committee completed the ASCO disclosure form prior to commencing the work on this guideline project. Further details about ASCO’s COI management are published in ASCOS’s COI management procedures summary (43).
Funding
Details in regards to guideline funding were not specified in the guideline publication (23).
Dissemination and implementation approach
The guideline was published in the Journal of Clinical Oncology (23) and is listed on ASCO’s website in the clinical guideline section (Table 3).
Planned update
Not specified in guideline document.
Associated resources
Slide sets, patient guide and decision aids are available from http://www.asco.org/institute-quality/asco-clinical-practice-guideline-update-chemotherapy-stage-iv-non-small-cell-lung.
British Thoracic Society (BTS) and Society for Cardiothoracic Surgery (SCTS) in Great Britain and Ireland
Introduction
The BTS and the SCTS in Great Britain and Ireland had developed a guideline on the radical management of patients with lung cancer in 2001 and decided to conduct an update of this guideline to provide comprehensive guidance on selection and risk assessment of suitable patients (Table 3) (25).
Guideline development methodology
The guideline development group determined the guideline scope based on the previous guideline and in consultation with members from both societies. A comprehensive literature search was performed and the evidence was assessed using the Scottish Intercollegiate Guidelines Network’s (SIGN) methodology. Recommendations were developed based on the evidence tables and graded according to SIGN. Research recommendations were also incorporated. The draft document was distributed amongst BTS and SCTS members and presented at society meetings for consultation and review. All feedback was assessed and reviewed by the guideline committee before the guideline was finalised, approved and published (25).
COI management
COI statements are included in the guideline publication (25).
Funding
The BTS funded all committee meetings (25).
Dissemination and implementation approach
The guideline was published in the Thorax journal (25) and is also disseminated through a link on the BTS website (Table 3).
Planned update
2013 (44).
Associated resources
A quick reference guide is available from http://www.brit-thoracic.org.uk/Portals/0/Guidelines/Lung%20Cancer/Guidelines/LungCancerQRG.pdf.
Cancer Care Ontario
Introduction
Cancer Care Ontario, a Canadian health government agency, has published nine clinical practice guidelines for lung cancer between 2008 and 2013 (Table 3) covering specific clinical questions in the area of non-small cell and small cell lung cancer management (26-34).
Guideline development methodology
At Cancer Ontario, working groups consisting of two to six clinicians or content experts and one Research Coordinator were established to produce each lung cancer guideline. The working groups determined the overall guideline topic, the individual clinical questions for each topic as well as the overall scope of each lung cancer guideline. The literature review process, that formed the basis of each guideline document, consisted of two stages: first, existing lung cancer guidelines were identified to see if an existing guideline could be formally adapted. If not, a systematic review of the evidence considering the highest level evidence was conducted. After the evidence was assessed and synthesised, the working groups developed the initial recommendations. The reasoning behind each recommendation and the degree of how much it is evidence-based versus expert consensus is explicitly stated in the recommendations. All draft guideline documents went through an internal and external review process. The external review process consisted of targeted peer review and professional consultation. The draft guideline documents were then revised by the individual working groups to assess and incorporate the feedback. The process and results that arose from the consultation review are documented in the final guideline documents. Cancer Care Ontario’s guideline development methodology is described in detail in the “Program in Evidence-Based Care Handbook” published by Cancer Care Ontario (45).
COI management
Working group authors had to declare COI as soon as they started on a guideline project and provide an update when the guideline was completed. The guideline chair and research coordinator were responsible to collate the declarations and updates and manage any conflicting interest according to Cancer Care Ontario’s COI policy (46). Reviewers also had to declare any competing interests.
Funding
Guideline development is supported by the Ontario Ministry of Health and Long-term Care through Cancer Care Ontario and editorially independent from its funding source (26-34).
Dissemination and implementation approach
The guidelines are published on Cancer Care Ontario’s website (Table 3) and indexed at National Guidelines Clearinghouse and CMA Infobase. In addition, the results of several systematic reviews are published in peer-reviewed journals (47-52).
Planned update
Each year the lung cancer guidelines are assessed with a document assessment tool developed by the Program in Evidence-Based Care at Cancer Ontario to determine if any guidelines are in need of an update (45).
Associated resources
Not identified.
Cancer Council Australia (CCA)
Introduction
CCA, a not for profit cancer charity, produces evidence-based clinical practice guidelines in oncology for the Australian health care context. In 2010, CCA was commissioned by Cancer Australia (an agency of the Australian Government) to update the lung guidelines originally published in 2004. The new web-based guideline covers treatment of non-small cell and small cell lung cancer, symptom management, supportive and palliative care (35).
Guideline development methodology
A multidisciplinary working group was established and the guideline objectives and scope were defined. Clinical questions according to PICO format were developed and systematic literature searches were carried out. The literature results were screened for relevance and formally assessed. The evidence was synthesised and analysed by the assigned working group members. Each question lead author developed the initial clinical question content, including formulation of evidence statements and draft recommendations and assigning the recommendation grades according to the NHMRC grading system (53). All draft content, including the recommendations and associated grades, was then internally reviewed and approved by all members of the working party before the draft guideline was released for public consultation. All externally received comments were considered by the working party and, where necessary, changes were made to the guideline. A formal response to each comment was documented. Once the guideline was finalized, it was published on CCA’s Cancer Guidelines Wiki (35). CCA’s Guideline Development Handbook provides a detailed description of the applied guideline development methodology (54).
COI management
COI statements were collected from each working group member at the start of the project. The management committee had the responsibility to collect and evaluate COI statements from all nominees. All working party members are responsible to provide updated COI statements if new interests arise (35).
Funding
Co-funding to develop these guidelines was received from Cancer Australia (35).
Dissemination and implementation approach
CCA’s clinical practice guidelines are available online via the CCA Cancer Guidelines Wiki (35). The link to the guidelines was distributed directly to relevant professional and other interested groups via email, print and social media campaigns as well as through meetings, national conferences and other CME events. By allowing guideline stakeholders to comment on guidelines content and submit new evidence on an ongoing basis, CCA is encouraging its stakeholders to engage with the guideline content on a long-term basis (54).
CCA is developing online learning modules to reinforce content knowledge for participants and support guideline uptake. CCA is going to pilot the development of a lung cancer QStream module originally developed by Harvard Medical School (54).
Planned update
Ongoing (54).
Associated resources
Online QStream module is in development (54).
CAP, IASLC and AMP
Introduction
Three professional societies, CAP, IASLC, and AMP, systematically reviewed the literature to develop an evidence-based guideline for selection of lung cancer patients for EGFR mutation and ALK rearrangement testing. The guideline addresses which patients and samples should be tested and when and how testing should be performed (36).
Guideline development methodology
A systematic literature review, including blinded screening for relevant studies, was performed. A formal quality assessment and data extraction was completed for all selected studies. Evidence tables were created. Based on the evidence assessment, content and evidence-based recommendations were formulated, evidence levels assigned and recommendation grades determined. In addition, recommendations based on formal expert consensus were added where appropriate and marked as such. The draft guideline then went through an extensive review process before it was finalised and published (36). The detailed methodological report is available from http://links.lww.com/JTO/A430 (55).
COI management
Before acceptance on the expert panel, all potential authors completed COI statements as per CAP’ procedures and were required to disclose new conflicts at each conference call. They had to submit a general updated COI form on a yearly basis (55). The COI statements are published with the guidelines.
Funding
The guideline development was jointly funded by CAP, IASLC and AMP (36).
Dissemination and implementation approach
The guideline is disseminated through the organisations’ websites and was released in Archives of Pathology & Laboratory Medicine, the Journal of Thoracic Oncology, and the Journal of Molecular Diagnostics (36).
Planned update
This guideline will be reviewed regularly, as mandated by publication of substantive and high-quality medical evidence that could potentially alter the original guideline recommendations (36).
Associated resources
A summary of recommendations is available from
http://www.cap.org/apps/docs/membership/cap_iaslc_amp_summary_of_recommendations.pdf. A patient guide is available from http://www.cap.org/apps/docs/membership/lc_patient_guide.pdf. A frequently asked question sheet is available from http://www.cap.org/apps/docs/membership/lc_faqs.pdf.
National Institute for Health and Care Excellence
Introduction
NICE is a UK-based health authority that provides national guidance and advice to improve health and social care. In 2011, NICE published a revision of the clinical practice guideline for lung cancer titled “Lung cancer. The diagnosis and treatment of lung cancer” (37).
Guideline development methodology
The methods that were used to develop the lung cancer guideline are in accordance with those set out by NICE in The guidelines manual (56). After the decision was made to update the lung cancer guideline, the guideline scope was defined and a lung cancer guideline development group was established. The group formulated clinical questions using the PICO framework where applicable. Comprehensive, systematic literature searches were carried out for each question and the evidence critically appraised and assessed. Health economic evidence was also included, assessed and synthesized. Based on the evidence synthesis, recommendations were developed and agreed upon by the working group. Qualifying statements about the strength of evidence, about the benefits and harms for the intervention being considered, the degree of consensus within the GDG and the costs and cost-effectiveness of an intervention were added. The guideline draft went through a consultation process, which was documented and published as a separate report on the NICE website. Based on the stakeholder comments, the guideline content was revised and went through a pre-publication check process, before the final guideline version was published (37).
COI management
At the start of the guideline development process, all COI statements from each guideline development group member were recorded. At each subsequent meeting, members declared any new, arising interests. For group members, that declared any conflicting interests, an evaluation took place and a management plan was implemented (37). The code of practice for declaring and dealing with conflicts of interest outlines the COI management procedures in further detail (57).
Funding
NICE commissioned the National Collaborating Centre for Cancer to develop this guideline. The health economic analysis was conducted by the London School of Hygiene and Tropical Medicine and funded by the National Collaborating Centre for Cancer (37).
Dissemination and implementation approach
This guideline is disseminated as web-based and short and long PDF versions on the NICE website. Numerous implementation tools have been developed to facilitate guideline update (see under associated resources). The NICE guidelines manual outlines the guideline dissemination and implementation approach for NICE guidelines in detail (56).
Planned update
After three years, the guideline will be formally evaluated to assess if an update is required (37).
Associated resources
A short version of this guideline, containing the key priorities, key research recommendations and all other recommendations, and a Quick Reference Guide (QRG) are available from http://www.nice.org.uk/guidance/index.jsp?action=byID&o=13465.
The following implementation tools are available from http://www.nice.org.uk/guidance/index.jsp?action=byID&o=13465: baseline assessment tool, clinical audit tool, costing report, costing template, multiple guidance audit tool, slide set, online educational tool about referral in case of suspected lung cancer.
Discussion
Considerable resources have been spent internationally on the development of lung cancer guidelines. This review article highlights that health professionals specialising in the treatment of lung cancer, patients and other stakeholders have access to numerous clinical practice guidelines developed for different local contexts. As the major concern around clinical practice guidelines is around quality, especially rigour of development, validity of recommendations and editorial independence, guideline users are encouraged to formally assess the quality of any identified lung cancer guideline (58). The guideline quality assessment instrument Agree II provides a validated tool to complete such quality assessments (59).
It was not part of this review to analyse and compare recommendations across guidelines addressing the same areas, nevertheless we are aware that variation does exist. For example, in patients with stage I non-small cell lung cancer who cannot tolerate surgery, the ACCP recommends stereotactic body radiotherapy (SBRT) (21), whereas NICE recommends patients should be offered continuous hyperfractionated accelerated radiotherapy (CHART) (37). ACCP does not mention CHART at all (21); NICE offers no guideline on SBRT, but recommends that further research should be undertaken (37). Undertaking a detailed content comparison across the identified lung cancer guidelines and investigating any variations, may be a worthwhile project to emerge from this initial review. It would be of interest to know if the reasons for any variations are resource related (for example Alberta Health Services does not recommend CHART because it is unavailable there), or a result of regional/cultural preferences in practice (for example the level of therapeutic aggression or nihilism). An example of the latter is the ACCP guideline for patients who have undergone resection of an isolated brain or adrenal metastasis, that adjuvant chemotherapy is suggested (21), whereas NICE only recommends adjuvant chemotherapy for patients without metastatic disease (37). The international variation in cultural attitudes to what are reasonable levels of medical intervention (as suggested by this example) could present an obstacle to the ultimate development of truly universal guidelines.
Compiling an overview of available lung cancer guidelines also pinpoints general challenges in the area of guideline development. Lung cancer guidelines, that follow an international standard, are presented in a validated, uniform format and are published together with the results of independently performed quality assessments, are still a vision of the future, even though significant efforts have been made to provide standards, methodologies and presentation guidelines (1,60-62).
Successful dissemination and implementation of lung cancer guidelines is another challenging area (39,63,64). Even if high quality evidence-based guidelines are available, it does not guarantee successful uptake by health professionals. Guideline developers, health care organisations, and governments need to put adequate resources into guideline dissemination and implementation and follow multiple implementation strategies to maximise uptake (39). Further there are many competing sources of information on lung cancer management besides guidelines which are readily available to health professionals and consumers. Although they may lack the endorsement of respected learned societies, these other sources, usually web-based, having avoided a lengthy development process, may provide more up-to-date information than traditional guidelines, and so become the first port of call for the information seeker. Conversely, without the rigour under which the guidelines are produced, use of that approach might lead to acceptance of faulty information.
It is therefore critical to keep the guidelines current if they are to be relevant and well used. Collaborating on lung cancer guidelines internationally by sharing literature searches and assessments is considered an effective approach to reduce duplication of effort and help developers keep the existing guidelines current (65). We hope this review provides an information starting point to bring together potential future collaborators with a view to developing integrated, dynamic, so called “living guidelines”, which can then be adapted to suit the different cultural and organisational contexts.
Summary
The aim of this review article was to provide a comprehensive overview of available clinical practice guidelines in the areas of small cell and non-small cell lung cancer. 22 clinical practice guidelines produced by 12 organisations with varying scopes and developed for different regions were identified and key features summarised. Health professionals in the area of lung cancer have no shortage of guidelines to assist the clinical decision making process. Future research needs to focus more on dissemination, implementation, guideline adherence and their effect on disease outcome. It is hoped this article will be a useful resource for clinicians and other stakeholders to easily access these different guidelines and assess relevance to their own practice. We also hope it may lead to organisations to pool their resources to develop consistent, internationally relevant guidelines for what is, after all, a global disease.
Acknowledgements
Jutta Johanna von Dincklage and David Ball are members of Cancer Council Australia’s Lung Cancer Guidelines Working Party that developed Cancer Council Australia’s Clinical practice guidelines for the treatment of lung cancer (35). Gerard Silvestri serves as American College of Chest Physicians’ lung cancer panellist that developed the third edition of ACCP Lung Cancer Guidelines (21). In the last 12 months, David Ball has sat on advisory boards for Boehringer-Ingelheim and Lilly Oncology, and given a lecture at a Pfizer sponsored symposium. In all cases the honoraria for these services have been paid directly to his employing institution, Peter MacCallum Cancer Centre.
Disclosure: The authors declare no conflict of interest.
References
- IOM (Institute of Medicine). Clinical Practice Guidelines We Can Trust, The National Academies Press, Washington DC, 2011.
- National Health and Medical Research Council. Summary for developers, Procedures and requirements for meeting the 2011 NHMRC standard for clinical practice guidelines, Melbourne, 2011.
- Jaklitsch MT, Jacobson FL, Austin JHM, et al. The American association for thoracic surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. [PubMed]
- Brodowicz T, Ciuleanu T, Crawford J, et al. Third CECOG consensus on the systemic treatment of non-small-cell lung cancer. Ann Oncol 2012;23:1223-9. [PubMed]
- Crinò L, Weder W. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v103-v115. [PubMed]
- Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii56-64. [PubMed]
- Sørensen M, Pijls-Johannesma M, Felip E, et al. Small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v120-5. [PubMed]
- Pavlidis N, Hansen H, Stahel R. ESMO clinical practice guidelines: development, implementation and dissemination. Ann Oncol 2010;21:v7-8. [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [PubMed]
- De Marinis F, Rossi A, Di Maio M, et al. Treatment of advanced non-small-cell lung cancer: Italian Association of Thoracic Oncology (AIOT) clinical practice guidelines. Lung Cancer 2011;73:1-10. [PubMed]
- National Comprehensive Cancer Network. eds. Lung cancer screening (Version 1). Washington: National Comprehensive Cancer Network, 2013.
- National Comprehensive Cancer Network. eds. Non-small cell lung cancer (Version 2). Washington: National Comprehensive Cancer Network, 2013.
- National Comprehensive Cancer Network. eds. Small cell lung cancer (Version 2). Washington: National Comprehensive Cancer Network, 2013.
- Trigo Pérez JM , López PG, Font EF, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer: an updated edition. Clin Transl Oncol 2010;12:735-41. [PubMed]
- Alberta Provincial Thoracic Tumour team. eds. Non small cell lung cancer stage I. Alberta: Alberta Health Services, 2011.
- Alberta Provincial Thoracic Tumour team. eds. Non small cell lung cancer stage II. Alberta: Alberta Health Services, 2011.
- Alberta Provincial Thoracic Tumour team. eds. Non small cell lung cancer stage III. Alberta: Alberta Health Services, 2011.
- Alberta Provincial Thoracic Tumour team. eds. Non small cell lung cancer stage IV. Alberta: Alberta Health Services, 2011.
- Alberta Provincial Thoracic Tumour team. eds. Small cell lung cancer-extensive stage. Alberta: Alberta Health Services, 2011.
- Alberta Provincial Thoracic Tumour team. eds. Small cell lung cancer-limited stage. Alberta: Alberta Health Services, 2011.
- American College of Chest Physicians. Diagnosis and management of lung cancer, 3rd edition: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013;143.
- Rodrigues G, Videtic GMM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: an American society for radiation oncology evidence-based clinical practice guideline. Pract Radiat Oncol 2011;1:60-71.
- Azzoli CG, Temin S, Aliff T, et al. 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol 2011;29:3825-31. [PubMed]
- American Society of Clinical Oncology. eds. American Society of Clinical Oncology guideline procedures manual (Expert Panel Version 4.0). Alexandria: American Society of Clinical Oncology, 2011.
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the Radical Management of Patients with Lung Cancer. Thorax 2010;65:iii1-27. [PubMed]
- Goffin J, Coakley N, Lacchetti C, et al. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer. Toronto (ON): Cancer Care Ontario; 2010 [In review 2012]. Program in Evidence-based CareEvidence-Based Series No.: 7-10 Version 2.2010.
- Ung YC, Bezjak A, Coakley N, et al. Positron emission tomography in radiation treatment planning for lung cancer. Toronto (ON): Cancer Care Ontario, 2010. Program in Evidence-based Care Evidence-Based Series No.: 7-18.
- Members of the Lung Cancer Disease Site Group. Postoperative adjuvant radiation therapy in stage II or IIIA completely resected non-small cell lung cancer Okawara G, Tey R, reviewers. Toronto, ON: Cancer Care Ontario, 2012. Program in Evidence-based Care Evidence-Based Series No.: 7-1-1 Version 2.
- Members of the Lung Cancer Disease Site Group. Use of preoperative chemotherapy with or without postoperative radiotherapy in technically resectable stage IIIA non-small cell lung cancer. Toronto (ON): Cancer Care Ontario, 2013. Program in Evidence-based Care Practice Guideline Report No.: 7-4 Version 2.
- Members of the Lung Cancer Disease Site Group. Altered fractionation of radical radiation therapy in the management of unresectable non-small cell lung cancer. Toronto (ON): Cancer Care Ontario, 2012. Program in Evidence-based Care Practice Guideline Report No.: 7-12 Version 2.
- Noble J, Ellis P, Mackay JA, et al. Ellis P, Ismaila N, reviewers. Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer. Toronto (ON): Cancer Care Ontario, 2012. Program in Evidence-based Care Evidence-based Series No.: 7-19.
- Members of the Lung Cancer Disease Site Group. The role of combination chemotherapy in the initial management of limited-stage small-cell lung cancer. Laurie S, Souter L, reviewers. Toronto (ON): Cancer Care Ontario, 2012. Program in Evidence-based Care Evidence-based Series No.: 7-13-1 Version 2.
- Members of the Lung Cancer Disease Site Group. Chemotherapy for relapsed small cell lung cancer. Toronto (ON): Cancer Care Ontario, 2013. Program in Evidence-based Care Evidence-based Series No.: 7-17 Version 2.
- The Lung Cancer Disease Site Group. 18-Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer. Ung Y, Ismaili Nofisat, reviewers. Toronto (ON): Cancer Care Ontario, 2012. Program in Evidence-based Care Evidence-based Series No.: 7-20 Version 2.
- Cancer Council Australia Lung Cancer Guidelines Working Party. Clinical practice guidelines for the treatment of lung cancer. Available online:
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for egfr and alk tyrosine kinase inhibitors. Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823-59. [PubMed]
- National Collaborating Centre for Cancer. eds. Lung cancer. The diagnosis and treatment of lung cancer. London: National Institute for Health and Clinical Excellence, 2011.
- ADAPTE Collaboration. 2007. Manual for Guideline Adaptation Version 1.0. ADAPTE Collaboration.
- Francke AL, Smit MC, de Veer AJE, et al. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. Available online:
- Alberta Health Services. eds. Guideline utilization resource unit handbook (Version 2). Alberta: Alberta Health Services, 2013.
- Akobeng AK. Principles of evidence based medicine. Arch Dis Child 2005;90:837-40. [PubMed]
- Lewis SZ, Diekemper R, Addrizzo-Harris DJ, et al. Methodology for development of guidelines for lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013;143:41S-50S.
- American Society of Clinical Oncology. eds. Policy summary: conflict of interest management procedures for clinical practice guidelines. Alexandria: American Society of Clinical Oncology, 2008.
- Guidelines. Available online: http://www.brit-thoracic.org.uk/guidelines.aspx
- Cancer Care Ontario. eds. Program in evidence-based care handbook. Toronto: Cancer Care Ontario, 2011.
- Cancer Care Ontario. eds. Program in evidence-based care (PEBC) conflict of interest policy. Toronto: Cancer Care Ontario, 2011.
- Goffin J, Lacchetti C, Ellis PM, et al. First-Line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol 2010;5:260-74. [PubMed]
- Ung YC, Bezjak A, Coakley N, et al. Positron emission tomography with 18fluorodeoxyglucose in radiation treatment planning for non-small cell lung cancer: a systematic review. J Thorac Oncol 2011;6:86-97.
- Okawara G, Ung YC, Markman BR, et al. Postoperative radiotherapy in stage II or IIIA completely resected non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer 2004;44:1-11. [PubMed]
- Noble J, Ellis PM, Mackay JA, et al. Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2006;1:1042-58. [PubMed]
- Cheng S, Evans WK, Stys-Norman D, et al. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2007;2:348-54. [PubMed]
- Ung YC, Maziak DE, Vanderveen JA, et al. 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J Natl Cancer Inst 2007;99:1753-67. [PubMed]
- NHMRC. eds. NHMRC additional levels of evidence and grades for recommendations for developers of guidelines. Canberra: NHMRC, 2009.
- Cancer Council Australia. eds. Development of clinical practice guidelines using Cancer Council Australia’s cancer guidelines wiki. Handbook 2012 for section authors and the guideline working party. Sydney: Cancer Council Australia, 2012.
- Lindeman NI, Cagle PT, Beasley MB, et al. Supplemental digital content. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the college of American pathologists, international association for the study of lung cancer and the association for molecular pathology. Available online:
- National Institute for Health and Care Excellence. eds. The guidelines manual. London: National Institute for Health and Clinical Excellence (NICE), 2012.
- National Institute for Health and Care Excellence. eds. A code of practice for declaring and dealing with conflicts of interest. London: National Institute for Health and Clinical Excellence (NICE), 2008.
- Alonso-Coello P, Irfan A, Solà I, et al. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care 2010;19:e58. [PubMed]
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839-42. [PubMed]
- Qaseem A, Forland F, Macbeth F, et al. Guidelines international network: toward international standards for clinical practice guidelines. Ann Intern Med 2012;156:525-31. [PubMed]
- Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008;337:a744. [PubMed]
- Treweek S, Oxman A, Alderson P, et al. Developing and evaluating communication strategies to support informed decisions and practice based on evidence (DECIDE): protocol and preliminary results. Implement Sci 2013;8:6. [PubMed]
- Grimshaw J, Eccles M, Thomas R, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med 2006;21:S14-20. [PubMed]
- Grimshaw JM, Thomas RE, Maclennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8:iii-iv, 1-72. [PubMed]
- Comprehensive Cancer Centre the Netherlands. eds. International collaboration. Sharing knowledge and experience: an international collaboration on guideline development. Utrecht: Comprehensive Cancer Centre the Netherlands, 2013.


