Impact of thoracic surgery on esophageal motor function—Evaluation by high resolution manometry
Introduction
Postoperative pulmonary complications (PPC), including atelectasis and pneumonia, occur in up to 59% (1) of patients undergoing thoracic surgery. They are considered a major contributing factor for in-hospital lethality, which ranges from 2–5% and is related to the type of performed resection (lobectomy or pneumonectomy). While certain esophageal motility disorders (EMD) are known to promote chronic aspiration and secondary pneumonia (2), the effects of transient postoperative EMD remains unclear. Most studies just report general clinical outcomes of major resections such as pneumonectomy (3,4) or esophagectomy (5) without explicitly analyzing EMD. The only notable exception is dedicated research in lung transplantation, where acid reflux is known to be associated with decreased time to early allograft injury (6), which even prompted certain workgroups to routinely perform pre-transplant high resolution manometry (HRM).
While conventional esophageal manometry still represents the Criterion Standard in diagnosis of dysphagia and motility disorders (7), recent developments in HRM further improved our understanding of esophageal dysfunction and allowed for true quantitative and qualitative analyses (8-10). The current diagnostic criteria for esophageal pressure topography resulted in the Chicago Classification (CC) (11). The assessment of the breaks in the current CC of 2014 refers to the analysis of large breaks to verify the contraction pattern. The analysis of large breaks (>5 cm), on the other hand, continues to have clinical relevance. Patients complaining of dysphagia are often able to detect large disruptions in swallowing (12). According to the new features of the CC v3.0 (11), a fragmented peristaltic is used if DCI is normal and breaks >5 cm. Compared to conventional manometry, contractile integrity in HRM is associated with isobaric contours for 20 mmHg or 30 mmHg (12). Segmental peristaltic breaks in the 20-mmHg isobaric are associated with weak peristalsis and impaired bolus clearance (12). Beside measurement of breaks to assess peristaltic integrity, the distal contractile integral (DCI) further characterizes contractile force, calculated as integrated pressure throughout a defined swallow (13). The evaluation begins at the lower esophageal sphincter. An important module for diagnosing a function disorder of the esophagus is the EGJ relaxation. Because of the interplay of different anatomical components, the determination of the pressure at the LES is not directly determinable. Therefore, the parameter integrated resting pressure (IRP4s) was developed to distinguish a normal from a disturbed EGJ relaxation (14).
The aim of this prospective study was to explore modifications of esophageal motility, contractility and function after thoracic surgery.
Methods
The study was approved by institutional ethics committee of the Otto-von-Guericke-University in Magdeburg (No. 110/11) and written informed consent was obtained from all participants. Twelve patients who underwent thoracic surgery at University Department of Cardiac and Thoracic Surgery at the faculty of Medicine, University Hospital Magdeburg were selected to take part in this study. The health status of the patients was comparable due to strict exclusion criteria. Furthermore, patients with structural esophageal disease, congenital malformations of the esophagus (e.g., esophageal atresia), acquired functional disorders of the esophagus (e.g., achalasia), gastrointestinal or esophageal anamnesis were excluded from the study. In addition, patients with history of EMD such as connective tissue diseases (e.g., dermatomyositis), Parkinson’s disease or multiple sclerosis were not permitted. To detect primary esophageal motility disorder, the HRM was performed preoperatively. In addition to routine work-up, all patients underwent preoperative and postoperative (48 hours and 7 days) HRM and 24 hours combined multichannel impedance and pH analysis. 24-h MII-pH was performed during the day of the thoracic procedure to analyze potential perioperative gastroesophageal reflux episodes.
High resolution manometry
High resolution manometry was performed through a 36-channel solid-state catheter with pressure sensors at 10 mm intervals (Solar GI HRM, Medical Measurement Systems, MMS Enschede, Netherlands). After local anesthesia, the catheter was introduced transnasally by simultaneously swallowing water and subsequently fixed in the correct position. After five minutes to adapt to the catheter, upper esophageal sphincter (UES) and lower esophageal sphincter (LES) resting pressure were recorded. All patients were investigated in upright position with 20 swallows of water (10×5 mL, 10×10 mL) and five swallows with pieces of white bread (1 cm3). Each swallow was analyzed for a period of 20–30 seconds. At the end of examination multiple rapid swallows (MRS) were performed to detect dysfunctions of postdeglutitive inhibitions within the lower high pressure zone. All data was stored and analyzed using dedicated software (MMS Database software, MMS, Enschede, Netherlands). Mean resting UES and LES pressures were determined at the beginning of the study after five minutes without any swallows. Besides DCI, IRP4s, contractile front velocity (CFV) and distal latency were determined.
The transition zone (Figure 1) for each swallow was manually measured using the analysis software mouse tool. It was defined as distance (in cm) along the y axis from the most distal portion of the 20-mmHg isobaric contour of the striated muscle contraction to the most proximal portion of the 20-mmHg isobaric contour of the smooth muscle contraction in all swallows. The length of peristaltic breaks was quantified in the 20-mmHg isobaric contour using the contour tool provided by the dedicated software. Normal range for isobaric contour breaks was 0–20% of all swallows for >5 cm breaks (“large breaks”) and 0–30% of all swallows for 2–5 cm breaks (“small breaks”) (12). The contractility of each swallow was categorized as normal, hypotensive or hypertensive.

For each patient and investigation, a diagnosis according to CC criteria was formulated.
24-hours multichannel intraluminal impedance and pH monitoring
Acid exposure to the distal esophagus and bolus transport was measured with a disposable catheter (Impedance and pH-metry disposable catheter AHCZ61A/5, MMS, Enschede, The Netherlands) according to standard methodology. The catheter was positioned with the pH electrode five cm above the manometrically localized LES. In this position impedance signals were detected at 3, 5, 7, 9, 15 and 17 cm above LES. Patients were instructed to record occurrence of symptoms, as well as time and duration of meals, and time and duration of supine and upright position. Pathological gastroesophageal reflux was diagnosed in case of pathological Johnson-DeMeester Score (>14.7), pathological acid exposure time (>4.2%) or in case of >72 reflux episodes detected with MII. All measurements were made with a portable Omega 4P IR recorder (MMS, Enschede, The Netherlands) and evaluated by MMS software.
Statistical analysis
All statistical analyses were performed using SPSS© version 21.0 (SPSS Inc., Chicago, Illinois, USA). Non-parametric tests (Friedman test and post-hoc Wilcoxon Rank Sum test for paired data) were used to compare data between different time points (preoperative baseline, perioperative and postoperative follow up). Data was expressed as median (range) using 5th and 95th centile of value unless specified otherwise. The graphic images were generated by Origin® version 8.0 (OriginLab Corp. Northhampton, MA, USA). Results were considered statistically significant for P<0.05, two-sided.
Results
Patient characteristics
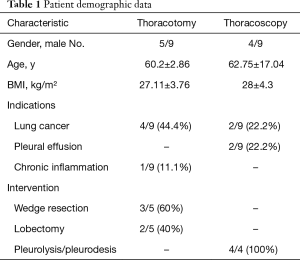
From our initial group of 12 patients (all male, age range 48–84 years) three were lost to follow-up due to relevant postoperative pain and unwillingness to further participate in the study. All relevant data are presented in Table 1.
Full table
Peri- and postoperative changes in esophageal motility
A total of 675 single swallows were analyzed by HRM. Perioperatively, DCI was significantly increased after 48 hours for 5 mL and solid swallows (5 mL, P=0.049; solid, P=0.014). After another week of postoperative follow-up, a significant drop of the DCI values was observed with normalization to preoperative baseline values (5 mL, P=0.039; solid, P=0.039, Figure 2). When comparing the measurements of 10 mL swallows, no significant differences can be determined. Perioperatively, corresponding changes of breaks in the contractile integrity were observed as well. Length of contractile breaks (5 mL, 10 mL water, solid) decreased perioperatively 48 hours after surgery. Similar to the changes in DCI, these changes returned to preoperative values within 7 days (Figure 3). The main part of contractile integrity interruptions was found in the proximal and mid-esophagus, within the transition zone and S1. Overall, the breaks in the proximal esophagus are larger than breaks in S2 and S3. Detailed information about values of the Chicago-Classification with perioperative changes were shown in Table 2.



Full table
Perioperative reflux pattern in 24-h MII-ph
Twenty-four hours MII-pH revealed abnormal gastro-esophageal reflux in only three patients with pathological DeMeester scores. The remaining six patients (66.7%) had normal results on pH-metry and MII analysis. There were no significant differences between patients who underwent thoracotomy or thoracoscopy. All relevant findings are presented in Table 3.

Full table
The results of each individual patient showed comparable changes in similar way of the manometric parameters and perioperative reflux pattern compared to other patients. The results are consistent in all patients.
Discussion
The aim of this study was to assess perioperative changes in esophageal motility. Therefore, we decided to analyze esophageal HRM preoperatively, postoperatively after 48 hours and after seven days. Only few publications deal with postoperative changes of esophageal motility after thoracic procedures, and in most of them HRM is performed six months or even longer after surgical interventions (15,16). For example Dougenis et al. analyzed oesophageal motility before and after pneumonectomy. They found an alteration of swallowing coordination and a significant increase of UES and LES relaxing pressure. Though only patients subjected to pneumonectomy were included (15). In addition, even fewer studies were aimed at directly comparing esophageal motility with a preoperative baseline investigation, as we did in our case-controlled design (15,16). This is particularly interesting as recent data suggests that acid reflux is associated with poorer outcomes after lung transplantation and that non-acid reflux may also induce a pulmonary inflammatory cascade, leading to acute and chronic rejection (6). A retrospective cohort analysis of 30 patients who underwent pre-transplant MII-pH confirmed that prolonged bolus clearance, increased total distal reflux episodes, and increased total proximal reflux episodes were all associated with decreased time to early allograft injury (6).
Current studies describe a DCI increase while swallowing bread (17,18), which was confirmed for liquid swallows by our data. Our results indicate, that there is no impaired esophageal motility following thoracic surgery. In contrast, we could demonstrate even hypercontractile changes in the direct perioperative period. Based on these observations, a surgically induced stimulatory effect on the autonomous nervous system can be assumed, leading to an imbalance between inhibitory and excitatory neurons. In concordance with these findings, severe physical stress is believed to play a crucial role in the pathogenesis of temporary esophageal hypercontractility (16).
In contrast to the hypercontractile changes in our study, esophageal dysfunction with hypomotility can be caused by severe anatomical and surgical changes such as vagal denervation, local ischemia, scar formation at the esophagus and mediastinum and by radical hilar and mediastinal lymphadenectomy (3). Basseri et al. observed a 6-fold increased risk for esophageal peristaltic dysfunction in lung transplant patients compared with healthy subjects (19). This is in line with data provided by Fiorelli and co-workers, who confirmed that pneumonectomy may cause significant EMD with reduction in LES resting pressure, especially when compared to lesser resections (20). Interestingly this did not lead to clinically relevant morbidity.
Data about breaks of the contractile integrity and their clinical relevance are sparse. Ribolsi et al. showed that large breaks are associated with a significant DCI decrease and simultaneously increase the bolus clearance time and duration of acid exposure. Patients with a pathological number of small interruptions also indicated decreases in DCI-values, but in this case the level of significance is not reached (21). The number and length of breaks is thus crucial for esophageal motility and bolus transport through the esophagus body.
In our study, all investigations were performed in a “physiological” upright body position. Various studies found significant changes in sitting position compared to lying posture, causing a reduction of DCI up to 69% (17,18,22). If our investigation would have been performed in supine position, potentially higher values could have been reached.
Similar to our results with “hypercontractile” perioperative changes of esophageal motility, Mohammed Khan investigated the impact of lung transplantation on esophageal motility with HRM and described the case of a temporary Jackhammer four weeks after major surgery. A measurement after 12 weeks showed a normalized esophageal pressure profile (16) which reflects the results of our investigation with a comparable “hypercontractile” perioperative esophageal motility.
Immediate perioperative complications were checked. Operative and anesthesia-related complications (re-thoracotomies, prolonged respiratory time) or emergency operations were not observed. However, this is not entirely comparable with regard to the limited number of cases. Cardiac arrhythmias or pneumonia did not be occurred. Besides, High resolution manometry was not performed during the surgery. A statement about HRM parameter changes cannot be made directly during the surgery and any missing complications.
Among other things, the surgical procedure in conjunction with the applied anesthesia (drugs, mechanical ventilation, patient support) can cause pathological gastroesophageal reflux. MII-pH allows an interpretation of the nature of the refluat (liquid, gaseous or mixed reflux), reflux symptom correlation, classification into subcategories of reflux and detection of non-acidic and acid bolus exposure time (23-25). The perioperative MII-pH measurement in our study showed numerous artifacts caused by intubation and ventilation during surgery also with increasing short and frequent acidic reflux episodes.
The patients received oral and sometimes subcutaneous morphine derivatives during surgery or postoperative. Opioids are known to affect esophageal motility. Among other things, opioids can cause a decreased LES relaxation, increased contraction amplitude and speed of contraction with simultaneous esophageal peristalsis (26). The literature describes changes in latency as a parameter for the rate of contraction by drugs that affect the smooth muscle. González et al confirmed these findings in prospective study with patients who received long-term morphine therapy investigated esophageal motility with the high resolution manometry. They described a LES-hypertonia and pathological relaxation in all cases similar to achalasia or functional EGJ obstruction (27). Ratuapli et al. were also able to demonstrate retrospective in a comparative study an opioid-induced EGJ outflow obstruction and spastic peristalsis in patients with continued opioid therapy compared to patients with at least 24 hours without pain therapy (28). A double-blind, cross-over study in healthy volunteers from Sweden showed that for example remifentanil induces dysfunction of esophageal motility with EGJ relaxation and distance latency decreasing (29). In the present work, the influence was not explicitly investigated.
So far, there is no study available that assesses the impact of surgical intervention on changes in length and exact localization of interruptions of the contractile integrity. Our data contributes to the understanding of esophageal motility, pathophysiology, contractility and function after thoracic surgery. We found a transient improved motility of the tubular esophagus postoperatively. The intrinsic intraesophageal reflex arcs appear to be unaffected by surgical intervention. These perioperative hypercontractile changes can be discussed as a protective mechanism in response to severe “stress”.
In light of our results, postoperative esophageal hypomotility is unlikely to promote secondary pulmonary complications. Further research is warranted.
Acknowledgements
The authors would like to thank the patients for their participation in this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- García-Miguel FJ, Serrano-Aguilar PG, Lopez-Bastida J. Preoperative assessment. Lancet 2003;362:1749-57. [Crossref] [PubMed]
- O'Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2013;19:5806-12. [Crossref] [PubMed]
- Suen HC, Hendrix H, Patterson G. Special article: Physiologic consequences of pneumonectomy. Chest Surgery Clinics of North America 2002;12:587-95. [Crossref] [PubMed]
- Vogt-Moykopf I, Zeidler D, Conradi T, et al. Functional disorders of the esophagus due to pneumectomy (Manomtric studies). Bruns Beitr Klin Chir 1970;218:1-11. [PubMed]
- D'journo XB, Michelet P, Avaro JP, et al. Complications respiratoires de l'oesophagectomie pour cancer. Rev Mal Respir 2008;25:683-94. [Crossref] [PubMed]
- Lo WK, Burakoff R, Goldberg HJ, et al. Pre-transplant impedance measures of reflux are associated with early allograft injury after lung transplantation. J Heart Lung Transplant 2015;34:26-35. [Crossref] [PubMed]
- Pandolfino JE, Kahrilas PJ. New technologies in the gastrointestinal clinic and research: Impedance and high-resolution manometry. World J Gastroenterol 2009;15:131-8. [Crossref] [PubMed]
- Pandolfino JE, Fox MR, Bredenoord AJ, et al. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil 2009;21:796-806. [Crossref] [PubMed]
- Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol 2008;103:27-37. [Crossref] [PubMed]
- Kahrilas PJ. Esophageal Motor Disorders in Terms of High-Resolution Esophageal Pressure Topography: What Has Changed?. Am J Gastroenterol 2010;105:981-7. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox MR, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Roman S, Lin Z, Kwiatek MA, et al. Weak Peristalsis in Esophageal Pressure Topography: Classification and Association With Dysphagia. Am J Gastroenterol 2011;106:349-56. [Crossref] [PubMed]
- Carlson DA, Pandolfino JE. The Chicago criteria for esophageal motility disorders. Curr Opin Gastroenterol 2012;28:395-402. [Crossref] [PubMed]
- Kahrilas PJ. Esophageal Motor Disorders in Terms of High-Resolution Esophageal Pressure Topography: What Has Changed? Am J Gastroenterol 2010;105:981-7. [Crossref] [PubMed]
- Dougenis D, Morrit G, Vagianos C, et al. Motility Disorders of the Esophagus before and after Pneumonectomy for Lung Carcinoma. Eur Surg Res 1996;28:461-5. [Crossref] [PubMed]
- Khan MQ, Nizami IY, Khan BJ, et al. Lung Transplantation Triggered "Jackhammer Esophagus": A Case Report and Review of Literature. J Neurogastroenterol Motil 2013;19:390-4. [Crossref] [PubMed]
- Zhang X, Xiang X, Tu L, et al. Esophageal Motility in the Supine and Upright Positions for Liquid and Solid Swallows Through High-resolution Manometry. J Neurogastroenterol Motil 2013;19:467-72. [Crossref] [PubMed]
- Sweis R, Anggiansah A, Wong T, et al. Normative values and inter-observer agreement for liquid and solid bolus swallows in upright and supine positions as assessed by esophageal high-resolution manometry. Neurogastroenterol Motil 2011;23:509-e198. [Crossref] [PubMed]
- Basseri B, Conklin JL, Pimentel M, et al. Esophageal Motor Dysfunction and Gastroesophageal Reflux Are Prevalent in Lung Transplant Candidates. Ann Thorac Surg 2010;90:1630-6. [Crossref] [PubMed]
- Fiorelli A, Vicidomini G, Milione R, et al. The effects of lung resection on physiological motor activity of the oesophagus. Eur J Cardiothorac Surg 2013;44:250-6. [Crossref] [PubMed]
- Ribolsi M, Balestrieri P, Emerenziani S, et al. Weak Peristalsis With Large Breaks Is Associated With Higher Acid Exposure and Delayed Reflux Clearance in the Supine Position in GERD Patients. Am J Gastroenterol 2014;109:46-51. [Crossref] [PubMed]
- Xiao Y, Read A, Nicodème F, et al. The effect of a sitting vs supine posture on normative esophageal pressure topography metrics and Chicago Classification diagnosis of esophageal motility disorders. Neurogastroenterol Motil 2012;24:e509. [Crossref] [PubMed]
- Sifrim D. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 2004;53:1024-31. [Crossref] [PubMed]
- Cho YK. How to Interpret Esophageal Impedance pH Monitoring. J Neurogastroenterol Motil 2010;16:327-30. [Crossref] [PubMed]
- Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology 2008;135:756-69. [Crossref] [PubMed]
- Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 2010;31:601-6. [Crossref] [PubMed]
- González ES, Bellver VO, Jaime FC, et al. Opioid-induced Lower Esophageal Sphincter Dysfunction. J Neurogastroenterol Motil 2015;21:618-20. [Crossref] [PubMed]
- Ratuapli SK, Crowell MD, DiBaise JK, et al. Opioid-Induced Esophageal Dysfunction (OIED) in Patients on Chronic Opioids. Am J Gastroenterol 2015;110:979-84. [Crossref] [PubMed]
- Savilampi J, Magnuson A, Ahlstrand R. Effects of remifentanil on esophageal motility: a double-blind, randomized, cross-over study in healthy volunteers. Acta Anaesthesiol Scand 2015;59:1126-36. [Crossref] [PubMed]


