Management of oral antiplatelet agents and anticoagulation therapy before bronchoscopy
Introduction
Oral anticoagulants are used to prevent and treat thromboembolic disease in patients with mechanical cardiac valve, atrial fibrillation (AF), stroke, deep venous thrombosis, and pulmonary emboli. For years, vitamin K antagonists (VKA) have been the main antithrombotic agents. Newer oral anticoagulation agents (NOAC) such as dabigatran, rivaroxaban, apixaban and edoxaban have been introduced into clinical practice starting in 2010 and have been adopted rapidly by the physicians (1,2). For AF the use of the NOAC has matched the use of warfarin in 2014 (1). Oral antiplatelet therapy such as aspirin and clopidrogel is widely used to prevent arterial thrombosis such as heart attacks, strokes and in-stent thrombosis (3,4). It is estimated that over 30% of the adult population uses aspirin for primary or secondary prevention of cardiovascular disease (5). Thus, the practicing pulmonologist is likely to encounter a patient using at least one of these agents and requiring a bronchoscopy. Balancing the risk of bleeding during bronchoscopy to the risk of thrombosis while withholding these agents is the corner stone of the peri-procedural management of anticoagulation therapy. This article aims at summarizing the available evidence on management of platelet aggregation inhibitors, as well as anticoagulant prior to bronchoscopic interventions.
Assessment of bleeding risk during bronchoscopy
General considerations
The risk of bleeding depends on the type of bronchoscopic procedure performed (Table 1), the skill of the operator (22), as well as additional risk factors such as coagulopathy, uremia, liver disease, pulmonary hypertension and the anticoagulation/antiplatelet therapy used (23).

Full table
It is generally an accepted practice to correct thrombocytopenia of less than 50.000/µL, International normalized ration (INR) of more than 1.5 and partial thromboplastin time (PTT) of more than 50 seconds (11,24,25). However, robust data to back this approach is lacking. In fact, studies suggest no correlation between risk of bleeding with transbronchial biopsies and coagulopathy (26,27).
Uremia can cause platelet dysfunction. An increased risk of bleeding, up to 45% with transbronchial biopsies was reported in older studies (28,29). A later study demonstrated significantly lower bleeding rates (4%) in patients with renal dysfunction who received desmopressin (DDAVP) (30,31). Usually considered to be at least a relative contraindication, pulmonary hypertension may not increase the risk of bleeding with transbronchial biopsies either (32).
Compared to other endoscopic interventions, small volume of bleeding in the airway, which may not lead to hemodynamic instability, can still cause significant airway compromise. The severity of bleeding in bronchoscopic procedures is not standardized. Clinically significant bleeding was arbitrarily defined in prior studies as more than 20ml of blood present in lavage fluid (26), more than 50 mL (6), 100 mL (33), or the judgement of the bronchoscopist (34). Others, defined the grade of bleeding severity based on the intervention required to stop the bleeding: mild bleeding does not require endoscopic intervention, moderate bleeding stops within 3 min after endoscopic intervention (bronchial occlusion and/or instillation of cold serum), and severe bleeding cannot be controlled endoscopically, causing hemodynamic or respiratory instability, and making it necessary to halt the procedure (17,35).
Assessment of the risk of bleeding in various bronchoscopic procedures
Broncho-alveolar lavage, bronchial brushing, transbronchial and endobronchial biopsy
A review of Bronchoscopic procedures over a period of 9 years at the Cleveland clinic, showed significant bleeding in 0.83%, increasing to 1.9% with transbronchial biopsies (TBB) (11). In another large retrospective study of 4,273 bronchoscopies, which included 2,493 broncho-alveolar lavage (BAL) and 173 TBB, bleeding occurred in 0.12% of all bronchoscopies and in 2.8% of TBB (6).
In the original description of cytology brushing in 163 patients, only 14 patients (8.5%) had transient hemoptysis with no significant bleeding (35). Bronchial cytology brushing is usually used in conjunction with other diagnostic modalities, which have higher risk of bleeding, making it difficult to estimate its additional bleeding risk.
Endobronchial biopsy (EBB) adds to the bleeding risk of a diagnostic bronchoscopy with mild to moderate bleeding occurring in 0.45% (11). The risk is lower than the risk of TBB, presumably due to the ability to visualize and treat the lesion.
Endobronchial cryobiopsy was shown to improve the yield and decrease the number of biopsies needed for the diagnosis of endobronchial lesion. A randomized trial compared conventional forceps biopsy to flexible cryoprobe. 281 patients got endobronchial biopsies using forceps and 282 had biopsies performed using a flexible cryoprobe. There was no difference in the incidence of bleeding that required any intervention (17.2% vs. 18.2%) (12). The significantly higher rate of bleeding compared to other large series may be related to the higher incidence of endobronchial malignancy (95%).
Transbronchial cryobiopsy
Transbronchial cryobiopsy is rapidly gaining popularity as a diagnostic technique for the diagnosis of interstitial lung disease. The risk of bleeding seems somewhat higher than routine transbronchial biopsy. Estimates of bleeding come from small series of cases. In a series of 32 cases bleeding was moderate in 8/32 (25%) and was severe in 17/32 cases (53%) (18). A series of 33 patients reported mild or moderate bleeding in 9%, and 21% respectively (17). In a larger series of 74 patients, bleeding occurred in 16 patients (22%) (19). In a series of 25 patients, three patients (12%) experienced serious hemorrhage immediately after biopsy, including one patient who survived a life-threatening bleed (20). In a randomized controlled multicenter study, looking at the rate of bleeding with transbronchial cryobiopsy compared to forceps biopsy. The rate of clinically relevant bleeding was higher after the cryobiopsy procedures compared to the forceps biopsies (15.9% vs. 4.1%, P<0.05). No fatal bleeding complication occurred (21). Finally, a meta-analysis of 11 investigations for transbronchial cryobiopsy showed clinically relevant bleeding in 20.99% (36).
Endobronchial ultrasound with transbronchial needle aspiration (EBUS-TBNA)
EBUS-TBNA is generally considered low risk for airway bleeding (Table 1). One series reported 3,123 cases performed for staging and diagnosing lung cancer. EBUS-TBNA was performed 11,753 times (3.76/case). No cases of airway bleeding were reported (37). The ACQUIRE registry enrolled 1,317 patients at six hospitals. Airway bleeding requiring an intervention occurred in 0.2% only (7). A systematic review of 9,119 EBUS cases reported no airway bleeding complications (38). Another meta-analysis of EBUS-TBNA showed a complication rate of 0.15% in 1,299 cases (39).
Interventional bronchoscopic procedures
Interventional procedures are generally done with multimodality approach with a variety of therapeutic options available to control bleeding. These include electrocautery, laser, APC, balloon occluder as well as the large volume suction capacity and local compression capability of rigid bronchoscopy. As such the relatively low complication rate probably reflect the experience, skill of the operator and the setting in which it is done.
A large series of 775 rigid bronchoscopies performed at a university hospital found that the majority of patients had no complications. Most patients were being treated with multimodality approach for central airway obstruction. 51 patients (6.6%) hemorrhaged during the procedure. In 27 cases the bleeding was classified as moderate, in 4 cases, it was classified as severe (40).
In the ACCP AQuIRE registry data, 15 centers performed 1,115 therapeutic bronchoscopy procedures on 947 patients for the treatment of central airway obstruction. Six patients (0.5%) had bleeding requiring intervention (41).
Overall we consider bronchoscopic procedures for airway inspection, BAL and EBUS to have a low risk of bleeding. Endobronchial biopsy and TBB have an intermediate risk of bleeding. Endobronchial cryobiopsy, therapeutic bronchoscopy and transbronchial cryobiopsy have a high risk of bleeding (Table 1).
Management of anticoagulation in patients undergoing bronchoscopic procedure
In addition to evaluating the bleeding risk related to the specific bronchoscopic procedure, patients receiving anticoagulation therapy should have an assessment of the risk of thromboembolism associated with withholding anticoagulation therapy.
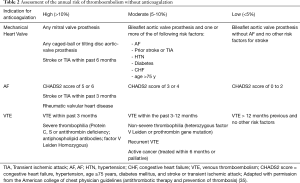
In its risk classification system, the ACCP classified patients annual risk of thromboembolism as high if more than 10%, moderate if 5–10% and low if it is less than 5% (42) (Table 2). This recommendation was based on historical studies outside the perioperative setting for patients who were not anticoagulated (42). However, in a study involving 1,185 patients, preoperative interruption of warfarin therapy (≤5 days), without bridging, was associated with low risk of thromboembolism (0.6%) in the following 30 days (43).
Full table
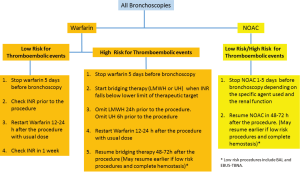
The British Thoracic Society guidelines recommend holding anticoagulation prior to bronchoscopy to minimize the perioperative risk of bleeding (23). The timing of interruption and the decision to bridge anticoagulation therapy depends on the risk of thromboembolic disease, the duration of action of the anticoagulant and the renal function (43,44) (Figure 1 and Table 3).

Full table
Peri-procedural management of VKA in elective bronchoscopy
When no bridging is required, VKA should be stopped 5 days prior to the procedure to achieve an INR of less than 1.5 the day of the procedure (42). Since the decay of the anticoagulation effect of warfarin may be delayed in elderly (44), INR can be checked the morning of the procedure (48), or the day before, to allow for correction of elevated INR with low dose (1–2.5 mg) oral vitamin K (50).
When bridging is necessary due to high risk of for thromboembolic events, subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin (UH) can be started when the INR falls below the therapeutic range. In high risk patients with an estimated creatinine clearance of less than 30 mL/min, the use of UH with close monitoring of the aPTT is the preferred option (48). It is recommended to stop LMWH for 24 hours prior to surgery and to hold UH for 4–6 hours prior to the surgery (42).
There are no specific data on when to resume anticoagulation after bronchoscopy. The recommendations are based on the perioperative management of anticoagulation before surgical procedures and gastrointestinal (GI) endoscopy. The 2013 ACCP guidelines recommend that post procedure, when hemostasis is achieved, warfarin can be resumed approximately 12 to 24 hours after the surgery (the evening of the procedure or next morning). If bridging is indicated heparin can be resumed 48–72 hours after the surgery, depending on the risk of bleeding post procedure (42). The British Thoracic Society guidelines recommend restarting warfarin the evening of the bronchoscopic procedure. Therapeutic dose LMWH should be omitted the day of the procedure and resumed the next day (23). Bridging therapy can be discontinued 5 days after starting warfarin and when the INR is in the therapeutic range (42).
The timing of resuming anticoagulation is based on the bleeding risk of the bronchoscopic intervention performed. High risk of bleeding has been defined as a risk of ≥1.5% for GI endoscopy (48). Given the much lower volume of bleeding required to cause significant airway compromise in the airway it is reasonable to adopt that as a minimum standard for bronchoscopy. We suggest that anticoagulation should be resumed later for procedure at higher risk from non-compressible biopsy sites such as TBB as compared to lower risk procedures such as BAL and EBUS-TBNA (Table 1).
Evidence behind bridging therapy
Only a few randomized controlled trial evaluated the use of bridging anticoagulation (51-53). In the BRIDGE study, a large randomized controlled trial in patients with atrial fibrillation (AF) undergoing an elective invasive procedure, forgoing bridging anticoagulation was not inferior to perioperative bridging with LMWH for the prevention of arterial thromboembolism. It resulted in fewer major bleeding episodes (1.3% vs. 3.2%; P=0.005) (53). Since only 3% of the patients had the highest CHADS2 scores of 5 and 6 and since these patients have an annual stroke risk of 12-18%,they would potentially still benefit from perioperative bridging anticoagulation (54). In a meta-analysis of 34 published studies, heparin bridging in patients receiving VKA was associated with higher rate of overall and major bleeding when compared to no bridging strategy. There was no difference in thromboembolic events (55). There are no studies assessing thromboembolic risk in high-risk patients with mechanical heart valve who had VKA interruption and did not receive heparin bridging (42).
The ACCP guidelines recommend bridging in patients with mechanical heart valve, AF or VTE when they are at high risk for thromboembolism. They recommend no bridging when these patients are at low risk for thromboembolism. When these patients are at moderate risk for thromboembolism an individualized approach is recommended (42).
Peri-procedural management of new oral anticoagulant (NOAC) in elective bronchoscopy
Currently there are four NOAC drugs approved by the Food and Drug Administration in the setting of AF and VTE. These include one oral direct thrombin inhibitor (Dabigatran) and three factor Xa inhibitors (Rivaroxaban, apixaban and edoxaban). They are given at a fixed dose and coagulation parameters do not need to be monitored (56).
Compared to warfarin, the NOAC have an immediate effect and shorter half-life (47). This allows them to be discontinued and resumed shortly prior and after the procedure. Bridging therapy is usually not thought to be necessary with these agents (56) and was not recommended by the European Heart Rhythm Association (45). The safety of performing invasive procedure in patients taking NOAC has been evaluated in the RE-LY (46), ROCKET AF (57) and the ARISTOTLE (58) trials.
In the RE-LY trial, Dabigatran had a similar periprocedural bleeding and thrombotic risk during elective, major and urgent procedures compared to warfarin. Early in the study, dabigatran was stopped 24 hours prior to the procedure; later on it was stopped from 2–5 days prior to high-risk procedure, depending on the patient’s renal function (Table 3) (46).
Schulman et al conducted a prospective cohort study to evaluate the safety of a pre-specified protocol. The protocol was slightly modified from the RE-LY trial, bridging was not used, and dabigatran was started after hemostasis was achieved. For minor procedures, 75 mg was used the evening of the procedure and increased to 110 or 150 mg the morning of the procedure. For increased risk of bleeding, the resumption time was delayed for 48-72h after the surgery and starting with the regular dose. The rate of major bleeding in that study was 1.8%, minor bleeding occurred in 5.2% and thromboembolism occurred in 0.2% (47).
In the ROCKET AF trial, rivaroxaban and warfarin interruption pre-operatively was compared in patients with non-valvular AF. Study drug was stopped >3 days prior to the procedure in 90% of the cases, the median duration of interruption was 6 days in the rivaroxaban group compared to 5 days in the warfarin group. Bridging therapy was used at the discretion of the investigator and occurred in 9% of patient. LMWH was the main agent used for bridging. The risk of thromboembolism and stroke were not different among warfarin and rivaroxaban (0.42% vs. 0.27%). The rate of major and non-major bleeding were 1% and 3% respectively. There was no difference in the rate of major bleeding or thromboembolic complications in patients who received bridging and those who did not (57).
Investigators from the ARISTOTLE trial reported the outcomes of peri-procedural management of patients taking apixaban and warfarin. When apixaban was interrupted preoperatively it was stopped 2–5 days before the procedure in most cases. Bridging therapy was given in 11.7% of the patients in both groups. For all procedures, whether the study drug was interrupted or not, there was no significant difference in the risk of stroke, systemic embolism or major bleeding. For procedures in which anticoagulation was interrupted, the rates of stroke or systemic embolism per procedure were 0.31% and 0.35% in apixaban and warfarin recipients, respectively. Major bleeding occurred in 1.62% of procedures in the apixaban group and 1.93% of procedure in the warfarin group. The authors did not report the effect of bridging therapy on complication rates (58).
The timing of discontinuation of direct factor Xa inhibitors such as rivaroxaban and apixaban as well as direct thrombin inhibitors such as dabigatran depend on the creatinine clearance (59). The data listed in Table 3 was taken from available trials looking at specific agents and from the manufactures recommendations (46,47,57,58). When a patient is undergoing a procedure at higher risk for bleeding, these medications may be withheld for a longer period of time (48).
There is no data on when to resume the NOAC after bronchoscopy, the recommendations are derived from patients undergoing surgical procedures and GI endoscopy. The full anticoagulation effect of the NOAC occurs shortly after administration they can be resumed 48h after the procedure in patients at high risk for bleeding (48). The European Heart Rhythm Association suggests that target specific oral anticoagulant may be restarted 6–8 hours after a procedure if there is immediate and complete hemostasis, but it notes that resumption in the first 48–72 hours may increase the risk of bleeding (45) (Figure 1).
We suggest that anticoagulation with NOAC should be resumed later for procedure at higher risk for bleeding such as TBB, as compared to lower risk procedures such as BAL and EBUS-TBNA (Table 1).
Emergent bronchoscopy and anticoagulation reversal
In the instance of hemoptysis or foreign body extraction requiring bronchoscopic intervention in patients on anticoagulation, it may need to be reversed. Protamine can reverse heparin completely and LMWH partially. Vitamin K, prothrombin complex (PCC) and fresh frozen plasma can be used to antagonize VKA. Due to its small infusion volume and rapid availability, PCC is the agent of choice for emergent reversal of VKA.
Recombinant factor VIIa has been used to antagonize dabigatran, idarucizumab (Praxbind) has been approved for dabigatran reversal. In the presence of renal insufficiency, hemodialysis can be used to remove dabigatran (60,61).
No specific reversal agents are currently available for the remaining NOAC. Unlike dabigatran, rivaroxaban, apixaban and edoxaban are not dialyzable (56). Although of limited effect, PCC is recommended in life threatening circumstances when patients are using rivaroxaban or apixaban. Plasmapheresis can be considered as well in these patient (56). Andexanet alfa is currently being studied for apixaban, rivaroxaban and possible edoxaban reversal (62).
Management of antiplatelet therapy in patients undergoing bronchoscopic procedure
Platelet inhibitors are increasingly used for coronary artery, peripheral vascular and neurovascular diseases. They are classified based on their mechanism of action. Aspirin blocks the synthesis of prostaglandins and thromboxane A2 from arachidonic acid, therefore inhibiting platelets aggregation. Dipyridamole inhibits the breakdown of cyclic AMP and prevents platelet activation. The P2Y12 receptor blockers include ticlopidine, clopidogrel, ticagrelor, prasugrel, and cangrelor, block the binding of adenosine diphosphate to P2Y12, inhibiting platelet activation.
Since several of these agents (such as aspirin, clopidogrel, and ticlopidine) irreversibly inhibit platelet function, their short half-life is clinically irrelevant. Once these agents are stopped, 10% to 14% of normal platelet function is restored per day (42). For agents with reversible platelet function inhibition (such as dipyridamole and cilostazol), the effect depends on the elimination half-lives.
Assessment of the risk of withholding antiplatelet therapy
The risk of thrombotic events depends largely on the underlying indication for the antithrombotic therapy. Such indication should be the primary factor in determining if it is safe to withhold this therapy for procedures considered to be at risk for bleeding. As discussed above the risk of bleeding varies based on the type of bronchoscopic procedure performed (Table 1).
Patients taking antiplatelet therapy for primary prevention of myocardial infarction or stroke are considered to be at low risk for thrombotic events. On the other hand, patients with recent placement of a coronary stent (within 3 to 6 months) or a recent myocardial infarction (within 3 months) are at high risk for a thrombotic event.
The American College of Chest Physicians recommends that patients receiving dual antiplatelet therapy for a coronary stent defer surgery for at least 6 weeks if a bare-metal stent is used and for at least 6 months if a drug-eluting stent is used (42). If surgery is needed within these time periods, the ACCP suggests continuing the dual antiplatelet therapy instead of stopping it 7 to 10 days before surgery. Even though the guidelines do not address patients undergoing bronchoscopies, one may extrapolate and conclude that stopping the dual antiplatelet therapy in such patients carries a high risk for a thrombotic coronary event.
Peri-procedural management of antiplatelet therapy in elective bronchoscopy
In a 2001 survey distributed at the American College of Chest Physicians meeting, aspirin was held by 71.5% of the respondents, while clopidogrel was held only by 61.3% prior to performing transbronchial lung biopsy via flexible bronchoscopy (63).
In 2002, Herth et al. evaluated the risk of bleeding associated with aspirin use during transbronchial lung biopsy in patients with no other coagulation problems (13). Bleeding severity was assessed based on the need for clinical intervention. Aspirin was not associated with an increased risk of bleeding, and the overall risk of severe bleeding was <1%. Subsequently, Ernst et al. prospectively evaluated the risk of bleeding associated with transbronchial biopsies in patients using clopidogrel with or without aspirin (14). Bleeding occurred in 89% and 100% of the patients, respectively. Even though no fatalities were observed in the study, all types of bleeding (minor/moderate/severe) were higher in the clopidogrel group.
EBUS-TBNA has been performed in patients who were on anti-platelet therapy. In a retrospective review of 409 EBUS- TNBA cases, 103 patients were on aspirin, 13 patients were on clopidogrel and 23 on both aspirin and clopidogrel. Overall bleeding events were low, occurring in 2.9% of all patients, 8.7% for patients on both aspirin and clopidogrel, 7.7% for clopidogrel alone, <1% for aspirin alone and 2.9% for patients not on any antiplatelet therapy. The difference was not statistically significant (P=0.164) (8). Another series reported twelve cases of EBUS-TBNA procedures performed on patients taking clopidogrel. Seven patients (66.7%) were taking aspirin in addition to clopidogrel. There was no significant bleeding seen in any cases at the time of bronchoscopy and no additional complications were identified during follow-up of at least 4 weeks (9). Martin et al. reported a series of fifteen patients on clopidogrel. A total of forty nodes were sampled. First, three passes per node were performed with a 22 gauge needle. If no complications were encountered, they followed with three passes with a 21-gauge needle. No significant bleeding occurred after the use of the 22 gauge or 21 gauge needles (10). Meena et al. reviewed 395 consecutive EBUS-TBNA. 37 patients were taking clopidogrel at time of biopsy. None were found to have significant bleeding (64).
The data regarding therapeutic bronchoscopies performed on antiplatelet agent is scarce. A large series report of multimodality therapeutic bronchoscopy done on anti-platelet therapy was reported by Harris et al. 108 patients had multimodality therapeutic bronchoscopy. 17 (15.7%) were taking aspirin and 91 (84.3%) were not on aspirin. The treatment modalities were similar in both groups except that more patients in the no aspirin group were treated with APC compared to the aspirin group (60.4% vs. 29.4%, P=0.031). The estimated blood loss between the aspirin and no aspirin groups was not significantly different. Overall, there was no difference in complications between both groups (16).
In the ACCP AQuIRE registry, 81 patients were reported to have high bleeding risk due to medications, but the agent used was not specified. There was no increase in the risk of complications or death in this group (41).
Table 1 list the rate of bleeding as reported with various bronchoscopic procedures while on aspirin, clopidogrel or both.
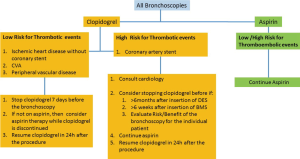
The British Thoracic Society guideline recommends stopping clopidogrel 7 days prior to endobronchial or transbronchial biopsies (23). The same guidelines recommend continuing the use of aspirin (Figure 2).
In patients with a high risk of coronary stent thrombosis, intravenous bridging may be considered, using the reversible glycoprotein inhibitors epifibatide or tirofiban (65). Cangrelor, a reversible intravenous P2Y12-inhibitor may also be used (66). Low molecular weight heparin have no role in these patients and should not be used.
Finally, in urgent cases when one cannot wait for the effect of the anti-platelet agent to wear off, the need for the procedure should be carefully weighed against the risk of bleeding.
Precautions to reduce the risk of bleeding when bronchoscopy is performed include the following: First, the procedure should be performed by a pulmonologist experienced in managing airway bleeding. Second, endotracheal blockers, balloon catheters and argon-plasma coagulation should be available to use in case of excessive bleeding. Third, deep sedation or general anesthesia should be considered for better control of the airway and to prevent traumatic laceration of the mucosa at the site of the procedure (67). Fourth, when endobronchial biopsy is planned, pre-injection of the biopsy site with epinephrine, use of direct probe or spray cryotherapy to freeze the biopsy site or cauterizing the area surrounding the biopsy site to decrease capillary blood supply to the biopsy site can be considered (68). When EBUS is performed, color Doppler prior to each pass is advised to avoid accidental puncture of blood vessels and the most distal target in the airway should be sampled first (assuming it does not affect the nodal staging in patients with cancer) (67).
The appropriate timing to resume antiplatelet therapy after bronchoscopy is not defined. The maximal antiplatelet effect occurs within minutes of resuming aspirin. At maintenance dose, it takes 5 to 10 days to attain maximal platelet inhibition with clopidogrel (42). One should restart this therapy as soon as clinically considered safe, usually within 24 h for clopidogrel (48). Since prasugrel and ticagrelor have a rapid onset of action, caution is recommended when restarting these agents (69).
Conclusions
Bronchoscopy is a relatively safe procedure with low overall risk of bleeding. However, even small volume of bleeding in the airway can be catastrophic. With the emergence of newer diagnostic and therapeutic options such as EBUS-TBNA, cryobiopsy, etc. the pulmonologist should be familiar with the bleeding risk associated with these procedures. Additionally, newer anticoagulant and antiplatelet agents are being used more frequently. The bronchoscopist should be familiar with strategies to minimize the risk of bleeding and thromboembolic events such as interruption of therapy, bridging and timely resumption of anticoagulation therapy post procedure. Since robust data is lacking, careful risk and benefit analyses individualized to each patient is prudent. A multidisciplinary discussion may help decision making in complex clinical scenarios.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Barnes GD, Ageno W, Ansell J, et al. Recommendation on the nomenclature for oral anticoagulants: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:1154-6. [Crossref] [PubMed]
- Baik SH, Hernandez I, Zhang Y. Evaluating the Initiation of Novel Oral Anticoagulants in Medicare Beneficiaries. J Manag Care Spec Pharm 2016;22:281-92. [Crossref] [PubMed]
- Harrington RA, Hodgson PK, Larsen RL. Antiplatelet Therapy. Circulation 2003;108:e45-7. [Crossref] [PubMed]
- Capodanno D, Angiolillo DJ. Management of Antiplatelet Therapy in Patients With Coronary Artery Disease Requiring Cardiac and Noncardiac Surgery. Circulation 2013;128:2785-98. [Crossref] [PubMed]
- Stuntz M, Bernstein B. Recent trends in the prevalence of low-dose aspirin use for primary and secondary prevention of cardiovascular disease in the United States, 2012–2015. Prev Med Rep 2016;5:183-6. [Crossref] [PubMed]
- Pue CA, Pacht ER. Complications of fiberoptic bronchoscopy at a university hospital. Chest 1995;107:430-2. [Crossref] [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the aquire registry. Chest 2013;143:1044-53. [Crossref] [PubMed]
- Swiatek K, Guthrie R, Elliott J, et al. Antiplatelet therapy in patients undergoing ebus-tbna: Risk vs benefit. Chest 2016;150:1017A. [Crossref]
- Stather DR, MacEachern P, Chee A, et al. Safety of endobronchial ultrasound-guided transbronchial needle aspiration for patients taking clopidogrel: a report of 12 consecutive cases. Respiration 2012;83:330-4. [Crossref] [PubMed]
- Martin RT, Parks C, Sharaf C, et al. Safety Of EBUS/TBNA In Patients With Mediastinal And Hilar Adenopathy Receiving Clopidogrel. B22 THE GOLDEN GUN? ADVANCES IN INTERVENTIONAL BRONCHOSCOPY. Am Thoracic Soc 2014: A2503-A.
- Cordasco EM Jr, Mehta AC, Ahmad M. Bronchoscopically induced bleeding. A summary of nine years' Cleveland clinic experience and review of the literature. Chest 1991;100:1141-7. [Crossref] [PubMed]
- Hetzel J, Eberhardt R, Herth FJ, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J 2012;39:685-90. [Crossref] [PubMed]
- Herth FJ, Becker HD, Ernst A. Aspirin does not increase bleeding complications after transbronchial biopsy. Chest 2002;122:1461-4. [Crossref] [PubMed]
- Ernst A, Eberhardt R, Wahidi M, et al. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest 2006;129:734-7. [Crossref] [PubMed]
- Hue SH. Complications in Transbronchial Lung Biopsy. Korean J Intern Med 1987;2:209-13. [Crossref] [PubMed]
- Harris K, Kebbe J, Modi K, et al. Aspirin use and the risk of bleeding complications after therapeutic bronchoscopy. Ther Adv Respir Dis 2016;10:318-23. [Crossref] [PubMed]
- Hernández-González F, Lucena CM, Ramirez J, et al. Cryobiopsy in the diagnosis of diffuse interstitial lung disease: yield and cost-effectiveness analysis. Arch Bronconeumol 2015;51:261-7. [Crossref] [PubMed]
- Hagmeyer L, Theegarten D, Wohlschlager J, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J 2016;10:589-95. [Crossref] [PubMed]
- Ussavarungsi K, Kern RM, Roden AC, et al. Transbronchial Cryobiopsy in Diffuse Parenchymal Lung Disease: Retrospective Analysis of 74 Cases. Chest 2017;151:400-8. [Crossref] [PubMed]
- DiBardino DM, Haas AR, Lanfranco AR, et al. High Complication Rate after Introduction of Transbronchial Cryobiopsy into Clinical Practice at an Academic Medical Center. Ann Am Thorac Soc 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Hetzel J, Petermann C, Eberhardt R, et al. Transbronchial Cryobiopsy – Is the bleeding risk changed in comparison to transbronchial forceps biopsy? Eur Respir J 2016.48.
- Facciolongo N, Patelli M, Gasparini S, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis 2009;71:8-14. [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- Gasparini S. Conventional Biopsy Techniques. In: Ernst A, Herth FJF, editors. Principles and Practice of Interventional Pulmonology. New York, NY: Springer New York; 2013:151-63.
- Papin TA, Lynch JP 3rd, Weg JG. Transbronchial biopsy in the thrombocytopenic patient. Chest 1985;88:549-52. [Crossref] [PubMed]
- Bjørtuft O, Brosstad F, Boe J. Bronchoscopy with transbronchial biopsies: measurement of bleeding volume and evaluation of the predictive value of coagulation tests. Eur Respir J 1998;12:1025-7. [Crossref] [PubMed]
- Chhajed PN, Aboyoun C, Malouf MA, et al. Risk factors and management of bleeding associated with transbronchial lung biopsy in lung transplant recipients. J Heart Lung Transplant 2003;22:195-7. [Crossref] [PubMed]
- Zavala DC. PUlmonary hemorrhage in fiberoptic transbronchial biopsy. Chest 1976;70:584-8. [Crossref] [PubMed]
- Cunningham JH, Zavala DC, Corry RJ, et al. Trephine air drill, bronchial brush, and fiberoptic transbronchial lung biopsies in immunosuppressed patients. Am Rev Respir Dis 1977;115:213-20. [PubMed]
- Mehta NL, Harkin TJ, Rom WN, et al. Should Renal Insufficiency Be a Relative Contraindication to Bronchoscopic Biopsy? Journal of Bronchology & Interventional Pulmonology 2005;12:81-3.
- Jain P, Sandur S, Meli Y, et al. ROle of flexible bronchoscopy in immunocompromised patients with lung infiltrates*. Chest 2004;125:712-22. [Crossref] [PubMed]
- Diaz-Fuentes G, Bajantri B, Adrish M. Safety of Bronchoscopy in Patients with Echocardiographic Evidence of Pulmonary Hypertension. Respiration 2016;92:182-7. [Crossref] [PubMed]
- Kozak EA, Brath LK., Do . "screening" coagulation tests predict bleeding in patients undergoing fiberoptic bronchoscopy with biopsy? Chest 1994;106:703-5. [Crossref] [PubMed]
- Trulock EP, Ettinger NA, Brunt EM, et al. THe role of transbronchial lung biopsy in the treatment of lung transplant recipients. an analysis of 200 consecutive procedures. Chest 1992;102:1049-54. [Crossref] [PubMed]
- Richardson RH, Zavala DC, Mukerjee PK, et al. The use of fiberoptic bronchoscopy and brush biopsy in the diagnosis of suspected pulmonary malignancy. Am Rev Respir Dis 1974;109:63-6. [PubMed]
- Sharp C, McCabe M, Adamali H, et al. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease-a systematic review and cost analysis. QJM 2017;110:207-14. [PubMed]
- Çağlayan B, Yilmaz A, Bilaceroglu S, et al. Complications of Convex-Probe Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: A Multi-Center Retrospective Study. Respir Care 2016;61:243-8. [Crossref] [PubMed]
- von Bartheld MB, van Breda A, Annema JT. Complication rate of endosonography (endobronchial and endoscopic ultrasound): a systematic review. Respiration 2014;87:343-51. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Drummond M, Magalhães A, Hespanhol V, et al. Rigid Bronchoscopy: Complications in a University Hospital. Journal of Bronchology 2003;10:177-82. [Crossref]
- Ost DE, Ernst A, Grosu HB, et al. Complications Following Therapeutic Bronchoscopy for Malignant Central Airway Obstruction: Results of the AQuIRE Registry. Chest 2015;148:450-71. [Crossref] [PubMed]
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: american college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e326S-e50S.
- Garcia DA, Regan S, Henault LE, et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med 2008;168:63-9. [Crossref] [PubMed]
- Hylek EM, Regan S, Go AS, et al. Clinical predictors of prolonged delay in return of the international normalized ratio to within the therapeutic range after excessive anticoagulation with warfarin. Ann Intern Med 2001;135:393-400. [Crossref] [PubMed]
- Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17:1467-507. [Crossref] [PubMed]
- Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012;126:343-8. [Crossref] [PubMed]
- Schulman S, Carrier M, Lee AY, et al. Perioperative Management of Dabigatran: A Prospective Cohort Study. Circulation 2015;132:167-73. [Crossref] [PubMed]
- Baron TH, Kamath PS, McBane RD. Management of Antithrombotic Therapy in Patients Undergoing Invasive Procedures. New England Journal of Medicine 2013;368:2113-24. [Crossref] [PubMed]
- Lange CM, Fichtlscherer S, Miesbach W, et al. The Periprocedural Management of Anticoagulation and Platelet Aggregation Inhibitors in Endoscopic Interventions. Deutsches Ärzteblatt International 2016;113:129-35. [PubMed]
- Woods K, Douketis JD, Kathirgamanathan K, et al. Low-dose oral vitamin K to normalize the international normalized ratio prior to surgery in patients who require temporary interruption of warfarin. J Thromb Thrombolysis 2007;24:93-7. [Crossref] [PubMed]
- Bajkin BV, Popovic SL, Selakovic SD. Randomized, prospective trial comparing bridging therapy using low-molecular-weight heparin with maintenance of oral anticoagulation during extraction of teeth. J Oral Maxillofac Surg 2009;67:990-5. [Crossref] [PubMed]
- Birnie DH, Healey JS, Wells GA, et al. Pacemaker or Defibrillator Surgery without Interruption of Anticoagulation. N Engl J Med 2013;368:2084-93. [Crossref] [PubMed]
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med 2015;373:823-33. [Crossref] [PubMed]
- Wight JM, Columb MO. Perioperative bridging anticoagulation for atrial fibrillation—the first randomised controlled trial. Perioperative Medicine 2016;5:14. [Crossref] [PubMed]
- Siegal D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012;126(:1630-9.
- Daniels PR. Peri-procedural management of patients taking oral anticoagulants. BMJ 2015;351:h2391. [Crossref] [PubMed]
- Sherwood MW, Douketis JD, Patel MR, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation 2014;129:1850-9. [Crossref] [PubMed]
- Garcia D, Alexander JH, Wallentin L, et al. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood 2014;124:3692-8. [Crossref] [PubMed]
- Dzik WS. Reversal of drug-induced anticoagulation: old solutions and new problems. Transfusion 2012;52 Suppl 1:45S-55S. [Crossref] [PubMed]
- Stangier J, Rathgen K, Stahle H, et al. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010;49:259-68. [Crossref] [PubMed]
- Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596-605. [Crossref] [PubMed]
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013;19:446-51. [Crossref] [PubMed]
- Wahidi MM, Rocha AT, Hollingsworth JW, et al. Contraindications and safety of transbronchial lung biopsy via flexible bronchoscopy. A survey of pulmonologists and review of the literature. Respiration 2005;72:285-95. [Crossref] [PubMed]
- Meena N, Abouzgheib W, Patolia S, et al. EBUS-TBNA and EUS-FNA: Risk Assessment for Patients Receiving Clopidogrel. J Bronchology Interv Pulmonol 2016;23:303-7. [Crossref] [PubMed]
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and managementThe Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014;35:2383-431. [Crossref] [PubMed]
- Angiolillo DJ, Firstenberg MS, Price MJ, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: A randomized controlled trial. JAMA 2012;307:265-74. [Crossref] [PubMed]
- Karnyski J, Dhillon SS, Kumar A, et al. Endobronchial Ultrasound-guided Transbronchial Needle Aspiration while Receiving Aspirin and Clopidogrel: Is It Always Safe? Ann Am Thorac Soc 2015;12:1733-4. [PubMed]
- Harris K, Kebbe J. Endobronchial biopsies on aspirin and prasugrel. Heart Lung Circ 2015;24:e68-70. [Crossref] [PubMed]
- Abualsaud AO, Eisenberg MJ. Perioperative Management of Patients With Drug-Eluting Stents. JACC Cardiovasc Interv 2010;3:131-42. [Crossref] [PubMed]