The effects of long-acting β2-agonists plus inhaled corticosteroids for early reversibility in patients with airway obstruction
Introduction
Salbutamol is a short-acting β2-adrenergic receptor agonist (SABA) used for the treatment and assesement of the early reversibilty in patients diagnosed as having obstructive lung disease. Also, it was the first selective β2-receptor agonistto be marketed in 1968 (1). Today, the long-acting β2-agonists (LABAs) plus inhaled glucocorticoids are the first choice of therapy in patients with asthma and chronic obstructive pulmonary disease. LABAs have also been reported to act in the short term and they can used for detection of early reversibilty. The late reversibility is valuable in these cases and these patients are evaluated with an FEV1 alteration a few weeks after administration of inhaled glucocorticoid (2,3). We suprised that the efficacy of LABAs plus inhaled glucocorticoids has not been investigated for early reversibility test according to reported articles. We aimed to compare the efficacy of salbutamol and long acting β2-agonists plus inhaled glucocorticoids for early reversibility test in patients with airway obstruction.
Materials and methods
Patients admitted to the Samsun Medicalpark Hospital, Department of Pulmonary Medicine, Samsun, Turkey, and meeting the inclusion criteria specified below were evaluated:
- Symptomatic patients (cough, dyspnea, and/or wheezing);
- Obstructive breath sounds on chest auscultation;
- Presence of airway obstruction in spirometry (FEV1/FVC ≤70% of expected);
- Patients who had never used bronchodilators before, or;
- Patients who had not received short- or long-acting inhaled bronchodilator therapy within the recent 12 hours.
The study was performed in accordance with the ethical principles in the Good Clinical Practice guidelines, in addition to applicable local regulatory requirements, and the protocol was approved by local ethics review boards. All the patients read the patient information form about the study procedure, and written informed consents were obtained.
Pulmonary function test and reversibility assessment
The basal FEV1 and FEV1/FVC values were measured using the MIR MiniSpir PC-Based USB Spirometer by the same physician following a 30-min resting period in an outpatient clinic setting. The test must be performed in the seated position, when the nose is clamped and nasal respiration is hindered. The patients performed the forced expiratory maneuver at least three times and the maximum FEV1 value was recorded as the basal value.
Reversibility test
The patients were randomized into four groups according to receiving bronchodilator drug. Following baseline spirometry, subjects inhaled salmeterol/fluticasone (FTC/SAL; Respiro® inhaler, Deva, Turkey; 25/250 mcg =2 inhalation; total dose 50/500 mcg) administered using a pressurised metered-dose inhaler with a spacer, formoterol/budesonide (BUD/FOR; Symbicort Forte® Turbuhaler®, AstraZeneca, Sweden; 320/9 μg), dry powder inhaler, beclomethasone dipropionate/formoterol (BDP/FOR; Foster®; Chiesi, Italy; 100/6 mcg =2 inhalation; total dose 200/12 mcg) administered using a pressurised metered-dose inhaler with a spacer or salbutamol (SLB; Ventolin®, GlaxoWellcome, 200 μg =2 inhalation; total dose 400 μg) administered using a pressurised metered-dose inhaler with a spacer and then spirometry was performed 15 min later. The bronchodilator drugs dosages were adjusted according to weight in patients with under 18 years old. Reversibility levels were evaluated as the absolute change in FEV1 and the percentage of change from the initial FEV1, calculated as FEV1%Δinit: post FEV1 – pre FEV1/pre FEV1 ×100 (according to American Thoracic Society guidelines) and bronchialreversibility is defined as a drug-inducedincrease in FEV1 of ≥200 mL and ≥12% baseline.
Statistical assessment
Results are presented as means ± standard errors of means. P<0.05 was considered significant. Descriptive group data were compared using the unpaired Student t-test and Pearson chi-square test.
Results
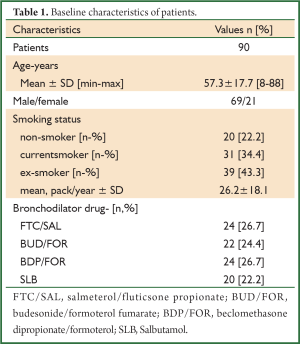
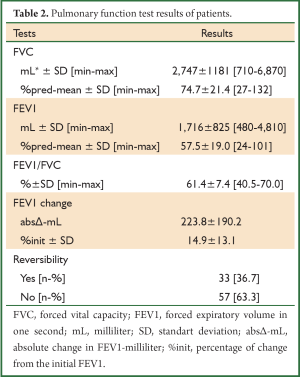
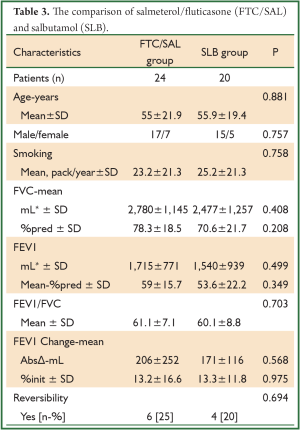
A total of 90 patients were evaluated, consecutively. The patients’ demographic data and baseline pulmonary function test results are shown in Tables 1,2. The mean age of patients was 57.3±17.7 (range, 8-88) years and the male-to-female ratio was 69/21. The baseline pulmonary function test results were mean FVC; 2,747±1,181 mL and 74.7%±21.4%, mean FEV1; 1,716±825 mL and 57.5%±19.0%, mean FEV1/FVC; 61.4%±7.4%. The bronchodilator drugs given before reversibility testing were as FTC/SAL, BUD/FOR, BDP/FOR and SLB in 24, 22, 24 and 20 patients, respectively. The reversibility was positive in 33 (36.7%) patients. The absolute change and percentage of change in FEV1 were 223±190 mL and 14.9%±13.1%, respectively. We aimed to compare the early bronchodilator effects of LABAs plus inhaled steroids and salbutamol. The comparison of FTC/SAL and SLB are shown in Table 3. There was no significant difference in demographic data and baseline pulmonary function test results. The absolute change and percentage of change in mean FEV1 were 206±252 mL, 13.2%±16.6% for FTC/SAL group and171±116 mL, 13.3%±11.8% for SLB group, respectively. And, it was not statistically significant. Also, the reversibility test was positive in 6 (25%) patients for FTC/SAL group and 4 (20%) patients for SLB group. There was no statistically significant for reversibilty results between FTC/SAL and SLB group.
Full Table
Full Table
Full Table
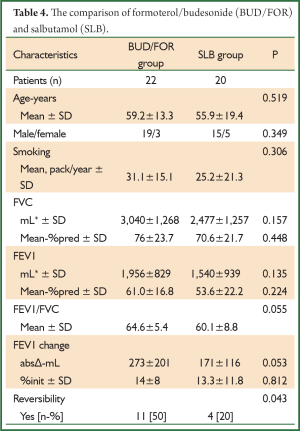
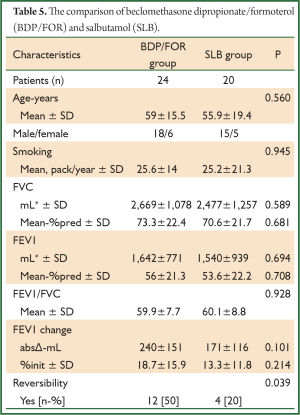
The comparison of BUD/FOR and SLB are shown in Table 4. There was no significant difference in demographic data and baseline pulmonary function test results. The absolute change and percentage of change in mean FEV1 were 273±201 mL, 14%±8% for BUD/FOR group, respectively. The absolute change of FEV1 was higher than SLB group but, it was not statistically significant. The patients with positive reversibility test was higher in BUD/FOR group 11 (50%) than SLB group 4 (20%), and it was statistically significant. The comparison of BDP/FOR and SLB ares hown in Table 5. There was no significant difference in demographic data and baseline pulmonary function test results. The absolute change and percentage of change in mean FEV1 were 240±151 mL, 18.7%±15.9% for BUD/FOR group, respectively. There was no statistically significant between two groups in change of FEV1. The patients with positive reversibility test was higher in BDP/FOR group 12 (50%) than SLB group 4 (20%), and it was statistically significant.
Full Table
Full Table
Discussion
The discussion section was limited due to lack of the any compatible article in literature. There is no consensus on which bronchodilator drug should be used for the reversibility test and the way in which it should be administered. The reversibility test may be performed using metered-dose inhaled bronchodilators (MDIs), dry powder inhaler, and MDIs through an air chamber or nebulizer. Short acting beta-2 agonists, salbutamol and terbutaline, are the most commonly used bronchodilators. Long-acting beta-2 agonist (LABA) drugs have also been reported to act in the short term. Lindberg et al. reported that the FEV1 at 5 minutes was compared in a randomized, double-dummy, cross-over single dose study of BUD/FOR 160/4.5 mcg, FTC/SAL 250/25 mcg, albuterol 100 mcg, and placebo (n=90). The change in FEV1 at all time points was greater with all active treatments vs. placebo. Improvement in FEV1 at 5 minutes was greater with BUD/FOR and albuterol than with FTC/SAL. At 180 minutes the FEV1 was significantly greater with both combination products compared to albuterol. Also they defined the time toperception of onset of effect was approximately 10 minutes earlier with BUD/FOR and albuterol compared to FTC/SAL (4).
Single doses of BUD/FOR 160/4.5 mcg (via Turbuhaler), BUD/FOR 320/9.0 mcg (via Turbuhaler) and FTC/SAL 250/50 mcg (via Diskus) werecompared in a cross-over fashion. As would be expected FEV1 at 3 minutes and average over 15 minutes post-dose was greater with both doses of BUD/FOR than FTC/SAL. Time to half maximum FEV1 and the time where a 15% increase in FEV1 within 60 minutes of the dose was observed were shorter with BUD/FOR (5). The onset of bronchodilation after a methacholine challenge was compared between BUD/FOR 160/4.5 mcg (1 or 2 inhalations via Turbuhaler) to FTC/SAL 250/50 mcg via Diskus and placebo in a cross-over study (n=27). Improvement in FEV1 3 minutes post-dose was faster with both doses of BUD/FOR compared to FTC/SAL. Median recovery times to 85% of baseline FEV1 were 3.3, 2.8, 8.9, and >30 minutes for BUD/FOR 160/4.5 mcg, BUD/FOR 320/9 mcg, FTC/SAL, and placebo respectively. Again, this is not unexpected as it is known that formoterol has a faster onset of action (6). The salmeterol + fluticasone combination was reported to provide an increase in FEV1 values 5 minutes after inhalation. Furthermore, an increase was reported in FEV1 values 5 minutes after inhalation of beclometasone + formoterol, high enough to meet the reversibility criteria (7). In our study, the bronchodilator effects of FTC/SAL and SLB by pMDI form were similar. We suggest that the FTC/SAL combinations shows the bronchodilator effects in 15 minutes and it may be used for the reversibility test instead of SLB in patients with airway obstruction. The BUD/FOR via turbuhaler® and BDP/FOR via pMDI combinations were more effective in the change of FEV1 than SLB within 15 minutes. Also, the patients with positive reversibility test were significantly higher in both of groups than SLB group and they may be used for the reversibility test instead of SLB in patients with airway obstruction.
However, our study is the first report stating that the LABA plus inhaled steroid combinations are effective in the early period of pulmonary function tests and that they may be used instead of short-acting bronchodilators in the early reversibility test. Similar clinical studies carried out with larger patient numbers would indicate whether our results are correct or not.
In conclusion, we think that performance of an early reversibility test using the combination of a long-acting beta-mimetic and an inhaled corticosteroid for treatment would enhance both the education of the patient in using the device and the reliability of the drug. We suggest that it may be used safely, not only for late reversibility but also for early reversibility and “you should make the reversibility test with Long-Acting β2-Agonists plus Inhaled Corticosteroids which used in treatment of obstructive lung diseases”.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bryan J. Ventolin remains a breath of fresh air for asthma sufferers, after 40 years. Pharm J 2007;279:404-5.
- BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax 1997;52:S1-28. [PubMed]
- Casaburi R, Adame D, Hong CK. Comparison of albuterol to isoproterenol as a bronchodilator for use in pulmonary function testing. Chest 1991;100:1597-600. [PubMed]
- Lindberg A, Szalai Z, Pullerits T, et al. Fast onset of effect of budesonide/formoterol versus salmeterol/fluticasone and salbutamol in patients with chronic obstructive pulmonary disease and reversible airway obstruction. Respirology 2007;12:732-9. [PubMed]
- Palmqvist M, Arvidsson P, Beckman O, et al. Onset of bronchodilation of budesonide/formoterol vs. salmeterol/fluticasone in single inhalers. Pulm Pharmacol Ther 2001;14:29-34. [PubMed]
- van der Woude HJ, Boorsma M, Bergqvist PB, et al. Budesonide/formoterol in a single inhaler rapidly relieves methacholine-induced moderate-to-severe bronchoconstriction. Pulm Pharmacol Ther 2004;17:89-95. [PubMed]
- Papi A, Paggiaro P, Nicolini G, et al. Beclomethasone/formoterol vs fluticasone/salmeterol inhaled combination in moderate to severe asthma. Allergy 2007;62:1182-8. [PubMed]


