Current concepts on coronary revascularization using BRS in patients with diabetes and small vessels disease
Introduction
Diabetes mellitus (DM) and small vessel (SV) disease are two major predictors of adverse outcome in patients treated by percutaneous coronary intervention (PCI) (1,2). These two conditions may frequently coexist, increasing even more the risk of adverse events after revascularization. Despite their undisputed benefit, current metallic drug-eluting stents (DES) remain associated with an increased incidence of adverse events when used in these settings. Moreover, metallic DES are associated with several drawbacks due to the persistence of their permanent struts in the vessel wall, such as: impaired vasomotion, vessel caging, jailing of collateral branches, and late lumen catch-up. To overcome these limitations, bioresorbable scaffold (BRS) technology has been recently developed, with the aim of providing, through the resorption process, the restoration of vascular physiology, positive remodelling of lumen and plaque shielding without precluding the possibility for late surgical revascularization (3). These potential advantages of BRS are particularly attractive in complex coronary artery disease (CAD), especially when distal or diffusely diseased coronary segments are involved and treated by PCI (4). First-generation BRS, largely represented by the poly-l-lactic acid (PLLA) ABSORB (Abbott Vascular, Santa Clara, CA, USA), demonstrated to be non inferior to contemporary metallic DES in terms of preventing 1-year restenosis (5). However, a trend towards increased early and midterm stent thrombosis (ST) was observed, especially when the scaffolds were used in off-label situations (e.g., implantation of oversized scaffold in vessel with diameter below 2.25 mm) (Ellis S.G. Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results, American College of Cardiology, 66th Annual Scientific Session, March 19, 2017, Washington DC; 4). Longer follow-up data have shown a two-fold increase in the target lesion failure (TLF) rate in the BRS arm of several trials (the 3-year ABSORB II and 2-year JAPAN and ABSORB III trials), largely driven by increased rates of target-vessel myocardial infarction (TV-MI) (6,7) (Ellis S.G. Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results, American College of Cardiology, 66th Annual Scientific Session, March 19, 2017, Washington DC). More recently, two other BRS received CE mark approval and became available for clinical use in Europe (DESolve from Elixir Medical, Sunnyvale, California, USA, and Magmaris from Biotronic, Bülach, Switzerland), but extensive outcome data are still lacking (8,9).
This review aims at summarizing current evidence on BRS in the subsets of DM and SV disease.
BRSs and DM
DM is a chronic metabolic disorder affecting up to 10–14% of the population in Western countries. DM is a major risk factor for the development of coronary artery atherosclerosis and affects around one-third of patients requiring PCI (10). Moreover, DM is one of the strongest among patient-related predictors of adverse outcome after percutaneous revascularization (1). Indeed, diabetic patients show increased platelet reactivity, inflammatory response and endothelial dysfunction (11). Moreover, insulin and insulin-like growth factors may accelerate smooth muscle cell proliferation after stenting by promoting stimulatory action on vascular smooth muscle cells (12). Even with the use of contemporary metallic DES, the presence of DM remains associated with increased risk of subsequent events, including myocardial infarction (MI), ST, restenosis and mortality (13-15). To note, clinical outcomes of diabetic patients treated by PCI are influenced by the severity of DM as assessed by the need of insulin treatment and by glycemic control (i.e., fasting glucose or glycated hemoglobin levels) (16,17).
Role of BRS technology in diabetic patients
The use of BRS in diabetic patients with CAD is particularly attractive for the following reasons: (I) current metallic DES still have higher risk of device-related adverse events in diabetic patients versus non-diabetics (18); (II) diabetic patients present frequently with diffuse coronary disease, a condition that might greatly benefit from successful vessel healing over long coronary segments; (III) patients with DM may require more frequently repeat percutaneous or surgical revascularizations, which might be facilitated by the complete resorption of previously implanted coronary stents. However, each of these theoretical advantages remains to be prospectically investigated. Moreover, DM is frequently associated to atherosclerosis of small coronary vessels, an angiographic setting that is emerging as particularly challenging for current BRSs.
Robust evidence related to the outcomes of BRS use in the context of DM is still lacking, since no dedicated randomized trial versus a metallic stent has been completed in this specific subset. Moreover, available outcome data in diabetics are limited to a single device type (Absorb BVS) and to mid-term follow-up (4). Several clinical trials and registries on the Absorb BVS have included diabetic patients, in variable proportions (Table 1). In a comparative analysis of 551 patients of ABSORB cohort B and ABSORB EXTEND, similar comparable outcomes were observed between diabetics and non-diabetics treated with Absorb BVS in term of 1-year TLF (3.7% vs. 5.1%, P=0.64) and definite/probable ST (0.7% in both groups) (19). To date, the largest study in patients with DM treated with Absorb BVS is a pre-specified, pooled analysis by Kereiakes et al. (20) including the ABSORB II, III, JAPAN trials and ABSORB EXTEND registry (22-25). In this study comprising 754 diabetic patients, the 1-year TLF rate was 8.3% vs. a pre-specified performance goal of 12.7% (P=0.0001). TLF were mainly driven by the 6.5% rate of target vessel MI. The 1-year rate of definite/probable ST was 2.3%, and the majority (1.3%) of these events occurred within 30 days. In this study, increasing age, insulin treatment and smaller pre-procedure reference vessel diameter (RVD) were independent predictors of 1-year TLF. When stratifying outcomes according to baseline RVD, diabetic patients with RVD <2.25 mm had two-times the incidence of TLF and TV-MI and three-times the incidence of ST compared to those with RVD ≥2.25 mm. The major impact of SVs with RVD <2.25 mm in diabetics is further emphasized by the fact that—limited to the three randomized trials included in this analysis—the 1-year TLF and device thrombosis rates for Absorb BVS vs. Xience were similar among patients with baseline QCA ≥ RVD 2.25 mm (1-year TLF 6.6% vs. 6.5%, P= NS; device thrombosis 1.3% vs. 0.4%, P= NS). It should be noted that the importance of RVD in most BRS trials is derived from analyses of QCA-based vessel sizing. However, QCA has important limitations in vessel sizing as compared to intravascular imaging modalities. In particular, QCA may prevent a reliable determination of native vessel size particularly when diffuse atherosclerosis is present and non-diseased segments are absent, thus leading to systematic RVD underestimation. Moreover, QCA-based estimation of the RVD is influenced by several procedural variables, such as the distance from X-ray tube and the vessel of interest (26). Therefore, a vessel sizing based on intravascular imaging modalities such as IVUS and OCT has been highly recommended when implanting BRS in complex/diffuse disease, which is a common feature of the diabetic subset (4). Related to the impact of insulin treatment, although insulin-dependent patients were less than one-third, they accounted for more than 50% of the scaffold thrombosis events. Conversely, the non insulin-dependent subgroup of this cohort had a 1-year rate of scaffold thrombosis of 1.5%, similar to both the 1.5% rate observed for the overall Absorb patients enrolled into the ABSORB III trial and the 1.4% rate observed in all Xience-treated diabetic patients in the ABSORB III trial (27). However, the weight of these findings by Kereiakes et al. is limited by several aspects: firstly, this analysis involved a population of patients with non-complex stable ischemic heart disease or stabilized acute coronary syndromes; secondly, for most investigators involved in the included trials this was the first-time clinical use of a BRS, with the inherent implications of the learning curve for an optimal implantation technique; thirdly, the study is limited to 1-year outcomes when the BRS resorption process is partial; most importantly, this analysis did not allow any direct comparison between the BRS and a metallic stent control. In this regard, a previous propensity-matched study by Muramatsu et al. investigated the clinical outcomes of patients with DM treated with either the Absorb BVS or the Xience EES by pooling individual data from ABSORB Cohort B and Absorb EXTEND with those of SPIRIT FIRST, II, III and IV studies (19). In this analysis, similar rates of 1-year device-oriented composite endpoint (DOCE) (3.9% vs. 6.4%, P=0.3) and device thrombosis were observed between the Absorb BVS (n=102 patients) and the Xience EES (n=172 patients). Similar results were observed when considering only the subgroup of insulin-dependent patients. Of note, in this study most patients of the propensity matched population had a rather low angiographic complexity, as indicated by the low prevalence of multivessel disease (25% in the BVS group) and of type B2/C lesions (39% in the BVS group). In the single-center experience by Wiebe et al. (21) in 120 patients in an “all-comers” setting including STEMI cases complex lesions (B2/C 60.6%), TLF rate was 8.9% and scaffold thrombosis was 2.7%, comparable to previous real-world cohort treated with 2nd generation DES. In a meta-analysis (5) comprising 3,389 non-diabetic and diabetic patients enrolled in four clinical trials (ABSORB II, ABSORB Japan, ABSORB China, ABSORB III) and randomized to receive a BVS or a Xience EES, DM was an independent predictor of TLF (OR 1.56, P=0.002), even if 1-year TLF was comparable between groups and the subgroup analysis showed a trend towards superiority of Xience in non-diabetics but not in diabetics. The ongoing SUGAR-EVE (EverolimuS-ElUtinG BioresorbAble VasculaR Scaffolds vErsus EVerolimus-Eluting Stents in Patients With DM, NCT02632292) and the COMPARE ABSORB (Bioresorbable Scaffold vs. Xience Metallic Stent for Prevention of Restenosis in Patients at High Risk of Restenosis, NCT02486068) are expected to provide further data on clinical outcomes in this specific subset. A dedicated diabetic sub-study of the COMPARE ABSORB trial will also investigate the potential benefit of Absorb BVS versus Xience in terms of IVUS-assessed plaque regression behind the stent struts at 5-year follow-up.

Full table
BRS technology for SV CAD
SV CAD is a frequently encountered angiographic finding, involving up to 20-30% of patients referred to the catheterization laboratory (28). Through literature, the atherosclerotic involvement of small coronary arteries has been defined in different ways, based on heterogeneous threshold values of RVD (frequently less than 2.75 mm, sometimes <2.5 or 3.0 mm) or stent diameters (usually less than 2.5 mm) (29). Several authors suggested the term of very SV (VSV) for those coronary segments with a RVD equal or lower than 2.25 mm (2).
Despite the great technical evolution of revascularization interventions, the optimal treatment of SV disease is still debated, as the risk of adverse outcomes bear an inverse correlation with vessel diameter (30). These patients are also predisposed to higher rate of clinical risk factors, and complex patterns of CAD including diabetes, renal failure and diffuse lesions (31). Compared to larger vessels, CABG is limited by high rates of technical failures and PCI is associated with an increased risk of restenosis and ST (2,32). Mechanisms suggested to explain that poorer outcomes include: (I) a high degree of vessels stretched and injury; (II) a small post-procedural lumen area; (III) a high metal density in case of metallic platform stent (MPS) implantation; (IV) a lower acute luminal gain with similar late loss when compared to large-vessel PCI and, consecutively; (V) a low threshold of cardiac ischemia due to neointimal hyperplasia (33-35). Currently, the cobalt-chromium everolimus-eluting stent (CoCr-EES) showed a better event-free survival rates in the setting of small coronary vessels compared to others MPSs, because of a low late loss, a stent platform with low strut thickness and high conformability to vessel (36). However, little is known regarding newer technologies as thin strut bioresorbable polymer stent, drug-eluting balloon (DEB) and the emerging bioresorbable devices (37-39). Given the relatively high restenosis rates even after last generation DES implantation, increasing interest has been focused on treatment of de novo small CAD with DEB. The 2-year follow-up of the BELLO (Balloon Elution and Late Loss Optimization) trial showed acceptable adverse events rate after treatment with IN.PACT Falcon™ paclitaxel DEB (Medtronic, Inc., Santa Rosa, California, USA) in coronary vessel with RVD lower than 2.8 mm, without evidence of late catch-up phenomenon requiring repeat intervention (40).
Role of BRS technology in SV disease
Outcome data in this setting are limited to the Absorb BVS device. The structural limitations of the current generation of Absorb BVS, like the strut thickness, the larger profile and the reduced deliverability, limit an extensive application of this technology in complex subsets, as that of SV CAD (41). To date, studies evaluating the clinical outcomes of this device in the setting of SV CAD are scant and mainly deriving from registries (Table 2, 42-48).
One of the first analysis from the ABSORB cohort B study showed at 2-year intravascular imaging follow-up a favourable balance between acute luminal gain and late lumen enlargement in vessels with lower RVD (<2.5 mm) (40). Accordingly, authors observed similar long-term clinical outcomes comparing the subset of patients with those with larger treated vessels in terms of MACE (7.3% vs. 10.2%, P=0.7) and ST (0% in both groups). Nevertheless, this analysis included simple lesions and, at the time when this study was conducted, only 3.0 mm BVS was available.
Tanaka et al. (43) assessed the performance of the Absorb BRS within a retrospective registry of 2 Italian high-volume centres (60 enrolled patients), comparing patients with 2.5 mm implanted scaffolds versus >2.5 mm. At 12 months follow-up, this registry showed a numerical, but not statistically significant, lower rate of TLR in smaller BRS patients (1.9% vs. 7.3%, P=0.2), despite a higher angiographic complexity. Similarly, a study by Wiebe et al. (44) in 101 “real world” patients found a similar 1-year TLR rate between the two BRS groups (15.7% in BRS =2.5 and 12.3% in BRS >2.5; P=0.5). In the BVS-Save Registry, the 12 months DOCE and the definite ST rates in the SV disease group (defined as a RVD <2.75 mm) were respectively 9% and 1.5% (44). As a matter of fact, the authors found a slightly higher occurrence of device failure if compared to studies related to last generation EES, but lower if compared with earlier devices (29,36). Notwithstanding, these results were limited by several aspects: their observational nature, the lack of a direct comparison with current CoCr-EES standard stent and the relatively small number of included patients. More recently, the outcomes data of bigger real-world patient populations were presented (Table 2, 46-48). The sub-study of the Italian RAI registry is the largest one focused on the interplay between BRS, vessel size, and outcome of patients enrolled in a prospective, multicenter registry (46). Compared to the previous studies, the BRS use was associated with lower DOCE and POCE rate, with a clinically driven target lesion and vessel revascularization of less than 4%, without statistically significant difference between small vs. non-SV group, at a median follow-up time of 14 months. However, authors found a numerically higher rate of mid-term scaffold thrombosis in SV patients that was late and very late and mainly driven by the VSV group. Other independent predictors of ST included DM and depressed ejection fraction (<45%) as already shown by others (49). Actually, recent findings based on available data from randomized BRS trials and observational registries showed that a meticulous implantation technique and selection of appropriate patients are required to optimize outcomes (50). Of note, the BRS implantation technique used in the RAI registry (mandatory pre-dilatation and strongly suggested post-dilatation) may have contributed to the lower TLF and early ST rates compared to previous BRS studies (51,52).
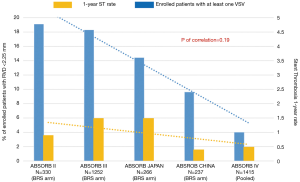
After the publication of the 1-year results of the ABSORB III trial, FDA representatives discouraged implanting the Absorb BRS in coronary vessels with RVD below 2.5 mm because of increased risk of TLF and ST (24). In that study, SV lesion (RVD <2.63 mm at QCA) treated by BRS trended to be associated with an increased rate of target lesion revascularization compared to Xience EES use (9.8% vs. 5.7%, P=0.09). Moreover, the 2-year results of the ABSORB III study showed a significant increase in TLF in the Absorb BVS group compared to controls (HR 1.42, 95% CI, 1.04–1.94, P=0.03) that became insignificant after cutting out patients with the smallest-caliber target vessels (HR 1.35, 95% CI, 0.93–1.96, P=0.12) (Ellis S.G. Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results, American College of Cardiology, 66th Annual Scientific Session, March 19, 2017, Washington DC). Part of this problem might be explained by bulky struts of scaffold (150 µm × 190 µm), limiting the effective flow area in small coronary lumens. Furthermore, in small coronary arteries subset, operators cannot comply with the correct sizing implantation rule, especially in case of RVD lower than 2.25 mm. As a matter of fact, Ishibashi et al. demonstrated that implantation of an oversized Absorb scaffold in a relatively SV may be associated with a higher risk of adverse events at long-term follow-up (53). The device expansion below nominal diameter could cause side-branch occlusion or microthrombus formation due to denser polymer surface pattern and a larger strut footprint. High pressure scaffold implantation might also play a role in strut embedment and ultimately influence the flow dynamic in the scaffolded segment. However, the oversized scaffold may produce vessel microperforation or dissection (53).
Accordingly, with a published letter, FDA reaffirmed the need for a meticulous attention to procedural details, advising health care providers of the observed Absorb BVS increased risk of events (Figure 1). Moreover, the manufacturer warned about scaffold deployment in the SV subset, strongly recommended the use of on-line QCA or intravascular imaging to confirm appropriate vessel sizing (RVD >2.5mm) and the need for adequate duration of dual-antiplatelet therapy. In Europe, even though the CE mark approval remains in place, the manufacturer restricted the Absorb BVS use only in centers participating in clinical registries. The ongoing Compare Absorb Trial will help to assess the role of PCI with Absorb BRS compared to Xience EES in patients at high-risk for restenosis due to clinical profile or coronary lesion complexity, including patients with target lesion RVD between 2.25–2.75 mm (54). Notwithstanding, the presence of complex lesion subset involving potentially BRS-unfavourable segments does not necessarily mean that the concept of “transient scaffolding” should be set aside. Accordingly, a metal free hybrid strategy of BRS for larger size vessels and DEB for smaller sized vessels could be considered respecting adequate implantation strategy (55).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Singh M, Gersh BJ, McClelland RL, et al. Clinical and angiographic predictors of restenosis after percutaneous coronary intervention. Circulation 2004;109:2727-31. [Crossref] [PubMed]
- Biondi-Zoccai G, Moretti C, Abbate A, et al. Percutaneous coronary intervention for small vessel coronary artery disease. Cardiovasc Revasc Med 2010;11:189-98. [Crossref] [PubMed]
- Indolfi C, De Rosa S, Colombo A. Bioresorbable vascular scaffolds - basic concepts and clinical outcome. Nat Rev Cardiol 2016;13:719-29. [Crossref] [PubMed]
- Tarantini G, Saia F, Capranzano P, et al. SICI-GISE Position paper: Use of Absorb BVS in clinical practice. G Ital Cardiol (Rome) 2016;17:28S-44. [PubMed]
- Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet 2016;387:1277-89. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479-91. [Crossref] [PubMed]
- Onuma Y, Sotomi Y, Shiomi H, et al. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial. EuroIntervention 2016;12:1090-101. [Crossref] [PubMed]
- Wiebe J, Dörr O, Ilstad H, et al. Everolimus- Versus Novolimus-Eluting Bioresorbable Scaffolds for the Treatment of Coronary Artery Disease: A Matched Comparison. JACC Cardiovasc Interv 2017;10:477-85. [Crossref] [PubMed]
- Haude M, Ince H, Abizaid A, et al. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur Heart J 2016;37:2701-9. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Available online: https://www.cdc.gov/diabetes/pdfs/data/2014-report-estimates-of-diabetes-and-its-burden-in-the-united-states.pdf
- Abizaid A, Kornowski R, Mintz GS, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol 1998;32:584-9. [Crossref] [PubMed]
- Bornfeldt KE, Raines EW, Nakano T, et al. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest 1994;93:1266-74. [Crossref] [PubMed]
- Kereiakes DJ, Cutlip DE, Applegate RJ, et al. Outcomes in diabetic and nondiabetic patients treated with everolimus- or paclitaxel-eluting stents: results from the SPIRIT IV clinical trial (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System). J Am Coll Cardiol 2010;56:2084-9. [Crossref] [PubMed]
- Stettler C, Allemann S, Wandel S, et al. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ 2008;337:a1331. [Crossref] [PubMed]
- D'Amico G, Fabris T, Mojoli M, et al. Impact of drug-eluting stent generation on patient- and stent-related adverse events of diabetic patients treated by percutaneous coronary intervention. Minerva Cardioangiol 2014;62:9-18. [PubMed]
- Akin I, Bufe A, Eckardt L, et al. Comparison of outcomes in patients with insulin-dependent versus non-insulin dependent diabetes mellitus receiving drug-eluting stents (from the First Phase of the Prospective Multicenter German DES.DE Registry). Am J Cardiol 2010;106:1201-7. [Crossref] [PubMed]
- Corpus RA, George PB, House JA, et al. Optimal glycemic control is associated with a lower rate of target vessel revascularization in treated type II diabetic patients undergoing elective percutaneous coronary intervention. J Am Coll Cardiol 2004;43:8-14. [Crossref] [PubMed]
- Stone GW, Kedhi E, Kereiakes DJ, et al. Differential clinical responses to everolimus-eluting and paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation 2011;124:893-900. [Crossref] [PubMed]
- Muramatsu T, Onuma Y, van Geuns RJ, et al. 1-year clinical outcomes of diabetic patients treated with everolimus-eluting bioresorbable vascular scaffolds: a pooled analysis of the ABSORB and the SPIRIT trials. JACC Cardiovasc Interv 2014;7:482-93. [Crossref] [PubMed]
- Kereiakes DJ, Ellis SG, Kimura T, et al. Efficacy and Safety of the Absorb Everolimus-Eluting Bioresorbable Scaffold for Treatment of Patients With Diabetes Mellitus: Results of the Absorb Diabetic Substudy. JACC Cardiovasc Interv 2017;10:42-9. [Crossref] [PubMed]
- Wiebe J, Gilbert F, Dorr O, et al. Implantation of everolimus-eluting bioresorbable scaffolds in a diabetic all-comers population. Catheter Cardiovasc Interv 2015;86:975-81. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2015;385:43-54. [Crossref] [PubMed]
- Kimura T, Kozuma K, Tanabe K, et al. A randomized trial evaluating everolimus-eluting Absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J 2015;36:3332-42. [Crossref] [PubMed]
- Ellis SG, Kereiakes DJ, Metzger DC, et al. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med 2015;373:1905-15. [Crossref] [PubMed]
- Abizaid A, Costa JR Jr, Bartorelli AL, et al. The ABSORB EXTEND study: preliminary report of the twelve-month clinical outcomes in the first 512 patients enrolled. EuroIntervention 2015;10:1396-401. [Crossref] [PubMed]
- Takagi K, Shannon J, Basavarajaiah S, et al. Discrepancies in vessel sizing between angiography and intravascular ultrasound varies according to the vessel evaluated. Int J Cardiol 2013;168:3791-6. [Crossref] [PubMed]
- Summaria F, Giannico MB, Masiero G, et al. The ABSORB III study. G Ital Cardiol (Rome) 2016;17:873-80. [PubMed]
- Biondi-Zoccai GG, Sangiorgi GM, Antoniucci D, et al. Testing prospectively the effectiveness and safety of paclitaxel-eluting stents in over 1000 very high-risk patients: design, baseline characteristics, procedural data and in-hospital outcomes of the multicenter Taxus in Real-life Usage Evaluation (TRUE). Int J Cardiol 2007;117:349-54. [Crossref] [PubMed]
- Cortese B, Bertoletti A, De Matteis S, et al. Drug-eluting stents perform better than bare metal stents in small coronary vessels: A meta-analysis of randomised and observational clinical studies with mid-term follow up. Int J Cardiol 2012;161:73-82. [Crossref] [PubMed]
- Keane D, Azar AJ, de Jaegere P, et al. Clinical and angiographic outcome of elective stent implantation in small coronary vessels: an analysis of the BENESTENT trial. Semin Interv Cardiol 1996;1:255-62. [PubMed]
- Akiyama T, Moussa I, Reimers B, et al. Angiographic and clinical outcome following coronary stenting of small vessels: a comparison with coronary stenting of large vessels. J Am Coll Cardiol 1998;32:1610-8. [Crossref] [PubMed]
- O’Connor NJ, Morton JR, Birkmeyer JD, et al. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation 1996;93:652-5. [Crossref] [PubMed]
- Farooq V, Gogas BD, Serruys PW. Restenosis: Delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv 2011;4:195-205. [Crossref] [PubMed]
- Briguori C, Sarais C, Pagnotta P, et al. In-stent restenosis in small coronary arteries. J Am Coll Cardiol 2002;40:403-9. [Crossref] [PubMed]
- Elezi S, Kastrati A, Neumann FJ, et al. Vessel Size and Long-Term Outcome After Coronary Stent Placement. Circulation 1998;98:1875-80. [Crossref] [PubMed]
- Hermiller JB, Rutledge DR, Mao VW, et al. Clinical outcomes in real-world patients with small vessel disease treated with XIENCE V® everolimus-eluting stents: One year results from the XIENCE V® USA condition of approval post-market study. Catheter Cardiovasc Interv 2014;84:7-16. [Crossref] [PubMed]
- Jinnouchi H, Kuramitsu S, Shinozaki T, et al. Two-year clinical outcomes of the NOBORI biolimus-eluting stents versus XIENCE/PROMUS everolimus-eluting stents in small vessel disease. Catheter Cardiovasc Interv 2016;88:E132-8. [Crossref] [PubMed]
- Wöhrle J, Markovic S, Rottbauer W, et al. Bioresorbable polymer sirolimus-eluting coronary stent compared with permanent polymer everolimus-eluting coronary stent implantation for treatment of small vessel coronary artery disease: CENTURY II trial. EuroIntervention 2016;12:e167-74. [Crossref] [PubMed]
- Giannini F, Latib A, Ancona MB, et al. A propensity score matched comparative study between paclitaxel-coated balloon and everolimus-eluting stents for the treatment of small coronary vessels. Catheter Cardiovasc Interv 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Naganuma T, Latib A, Sgueglia GA, et al. A 2-year follow-up of a randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels the BELLO study. Int J Cardiol 2015;184:17-21. [Crossref] [PubMed]
- Abizaid A, Ribamar Costa J Jr. Bioresorbable Scaffolds for Coronary Stenosis: When and How Based Upon Current Studies. Curr Cardiol Rep 2017;19:27. [Crossref] [PubMed]
- Diletti R, Farooq V, Girasis C, et al. Clinical and intravascular imaging outcomes at 1 and 2 years after implantation of absorb everolimus eluting bioresorbable vascular scaffolds in small vessels. Late lumen enlargement: does bioresorption matter with small vessel size? Insight from the ABSORB cohort B trial. Heart 2013;99:98-105. [Crossref] [PubMed]
- Tanaka A, Ruparelia N, Kawamoto H, et al. Clinical outcomes following bioresorbable scaffold implantation in small vessels. Int J Cardiol 2016;207:59-61. [Crossref] [PubMed]
- Wiebe J, Hoppmann P, Kufner S, et al. Impact of stent size on angiographic and clinical outcomes after implantation of everolimus-eluting bioresorbable scaffolds in daily practice: insights from the ISAR-ABSORB registry. EuroIntervention 2016;12:e137-43. [Crossref] [PubMed]
- Latini RA, Granata F, Ielasi A, et al. Bioresorbable vascular scaffolds for small vessels coronary disease: The BVS-save registry. Catheter Cardiovasc Interv 2016;88:380-7. [Crossref] [PubMed]
- Masiero G, Tarantini G, Mojoli M, et al. Bioresorbable vascular scaffold technology for small vessel coronary artery disease: results from the Italian multicenter RAI Registry. JACC 2016;68:B171-2. [Crossref]
- Ezhumalai B, Seth A. TCT-434 Implantation of Absorb Bioresorbable Vascular Scaffolds in Small Vessel Coronary Artery Disease: Long-term follow-up. JACC 2016;68:B175. [Crossref]
- Paradies V, Smits P, Royaards KJ, et al. TCT-422 6 year clinical outcomes after Absorb bioresorbable scaffold implantation in small vessel: The Maasstad Absorb Registry. JACC 2016;68:B170-1. [Crossref]
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Jama 2005;293:2126-30. [Crossref] [PubMed]
- Puricel S, Cuculi F, Weissner M, et al. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J Am Coll Cardiol 2016;67:921-31. [Crossref] [PubMed]
- Collet C, Asano T, Sotomi Y, et al. Early, late and very late incidence of bioresorbable scaffold thrombosis: a systematic review and meta-analysis of randomized clinical trials and observational studies. Minerva Cardioangiol 2017;65:32-51. [PubMed]
- Tarantini G, Mojoli M, Masiero G, et al. Clinical Outcomes of Overlapping Versus Non-overlapping Everolimus-eluting Absorb Bioresorbable Vascular Scaffolds: An Analysis From the Multicentre Prospective RAI Registry (ClinicalTrials.gov Identifier: NCT02298413). Catheter Cardiovasc Interv 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Ishibashi Y, Nakatani S, Sotomi Y, et al. Relation between bioresorbable scaffold sizing using QCA-Dmax and clinical outcomes at 1 year in 1,232 patients from 3 study cohorts (ABSORB Cohort B, ABSORB EXTEND, and ABSORB II). JACC Cardiovasc Interv 2015;8:1715-26. [Crossref] [PubMed]
- Smits P. ABSORB Bioresorbable Scaffold vs. Xience Metallic Stent for Prevention of Restenosis Following Percutaneous Coronary Intervention in Patients at High Risk of Restenosis (Compare Absorb). June 24, 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02486068
- Tanaka A, Jabbour RJ, Mitomo S, et al. Hybrid Percutaneous Coronary Intervention With Bioresorbable Vascular Scaffolds in Combination With Drug-Eluting Stents or Drug-Coated Balloons for Complex Coronary Lesions. JACC Cardiovasc Interv 2017;10:539-47. [Crossref] [PubMed]