Effectiveness and safety of the ABSORB bioresorbable vascular scaffold for the treatment of coronary artery disease: systematic review and meta-analysis of randomized clinical trials
Introduction
Routinary use of second generation drug eluting stents (DES) is nowadays a consolidate practice. Many studies provided evidence on efficacy and safety of these devices but warning concerns still arise from cardiological community regarding the increased risk of late and very late stent thrombosis (1). Several studies showed that the presence of several uncovered struts was related with the presence of persistent inflammatory reaction around the device (2-4). Moreover, after DES implantation the anatomy and physiology of the endothelium seem to remain abnormal, with increased vasoconstriction in response to some stimuli (e.g., acetylcoline) (5,6).
In this framework, the new bioresorbable vascular scaffolds (BVS) were introduced as a promising technology. Nevertheless, the efficacy and safety profile of BVS remains unclear. Such type of stent can potentially overcome the long-term limitations of permanent stent implantation, restoring physiological vasomotion and endothelial function of the treated vessel without precluding future surgical revascularization at the same lesion. Bioresorbable scaffolds consist of a polymer or bioresorbable metal alloy (7). Currently, several polymers are available, each with different chemical compositions, mechanical properties, and subsequently bioabsorbition times. The most frequently used polymer in the current generation of BVS is poly-L-lactic acid (PLLA), which form the backbone of the BVS device while the coating consists of poly D,L-lactide acid (PDLLA). PDLLA is a random copolymer of D and L-lactic acid with lower crystallinity (7) and it is responsible of the release of the anti-proliferative drug everolimus (7).
The current and most used BVS is the absorb BVS (Abbott Vascular, Santa Clara, CA, USA) which had been yet the subject of several randomized clinical trial (RCT) (8,9), which evaluated clinical outcomes from BVS implantation, but being underpowered to detect differences in treated lesion efficacy and to value safety endpoints. The aim of this review is to compare bioresorbable scaffold implantation versus everolimus drug eluting stent in term of efficacy and safety lesion related outcomes.
Methods
Search strategy
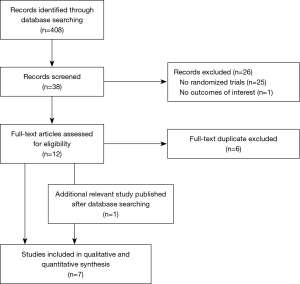
A systematic search of RCT comparing Absorb BVS versus durable polymer cobalt-chromium everolimus eluting stent (EES) was performed. The search was carried out in PubMed, Biomed Central, Google Scholar and the Cochrane library. The search was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) amendment to the quality of reporting of meta-analyses (QUOROM) statement (10-13). The search strategy terms included “BVS” and “absorb” and it was carried between January and March 2017. Two reviewers (MS, RP) analysed the records, valuing the ones deserving a full-text analysis (Figure 1).
Selection criteria
Inclusion criteria were:
- Studies with randomized design comparing the Absorb BVS versus durable polymer cobalt-chromium Everolimus Eluting Stent;
- Studies reporting the number of events for the outcomes of interest at 6-month follow-up and over: all-cause death, target lesion failure (TLF), cardiac death, target vessel myocardial infarction (MI), ischemia driven target lesion revascularization (ID-TLR), device oriented composite endpoints (DOCE), definite or probable device thrombosis (ST). DOCE was defined as the composite endpoint for TLF (cardiac death, ID-TLR, target vessel MI). The ST was defined according to the Academic Research Consortium (ARC) criteria (14);
- Study published in English.
Exclusion criteria were:
- Non-randomized trial (e.g., review, editorials, meta-analysis, case report/series, propensity score matching analysis, observational studies);
- Duplicate study, duplicate of the sample study;
- Follow-up duration less than 6-months;
- Comparison between BVS to a non-permanent polymer DES (e.g., bioabsorbable or bioresorbable polymer DES).
Data abstraction, endpoints, subgroup analyses
The reviewers completed a database with data regarding: the journal, year of publication, population characteristics and endpoint of interest. The endpoints of the analysis were the odds ratio (OR) of all-cause mortality, cardiac death, ischemia-driven target lesion revascularization, DOCE, target vessel MI, definite or probable device thrombosis. Subgroup analysis according to the length of the follow-up (≤1 vs. >1 year) was performed.
Internal validity and quality appraisal
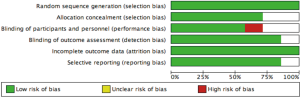
Two unblinded reviewers evaluated the quality of included studies. The presence of low or high risk of selection, analytical, adjudication, attrition and detection bias was evaluated. The divergences were resolved by consensus.
Statistical analysis
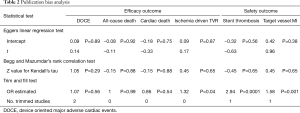
Continuous variables were summarized as mean (± standard deviation) or median (interquartile range) while the categorical ones were expressed as percentage or number. To convert median (interquartile range) to mean (standard deviation) we used formula accepted in the literature (15). The endpoints were expressed as OR. Statistical heterogeneity was appraised with I2 statistic. A value of I2 of 0 to 25% was considered insignificant, 26 to 50% low, 51 to 75% moderate, and >75% high (16). When the I2 was <50% a fixed effect model was used, while if the I2 was >50% a random effect model was chosen. The Chi2 test has been used to test the difference between sub-group analyses. Finally, meta-regression analysis was performed to weigh the effect of some potential confounding factors (demographical characteristics of the population, previous MI, previous percutaneous coronary intervention, cardiovascular risk factors, coronary artery treated, clinical presentation). Publication bias was evaluated by funnel plots analysis with Duval and Tweedie trim and fill, Begg and Mazumdar rank correlation, Egger’s regression intercept (17). Prometa software 3 (Internovi, Cesena, Italy) and RevMan 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) were the statistical software used.
Results
Search strategy
A total of 408 records were analysed (Figure 1). After a first evaluation of titles and abstracts 38 records were screened and 26 of these were excluded because they did not meet the inclusion criteria and 6 because they were duplicate (Figure 1). A total of seven studies were included in the meta-analysis (18-24), one of which was added after the publication in March 2017 (AIDA trial) (25). We also decided to use the data at two years, recently published in clinicaltrial.org for ABSORB III trial (26).
Population characteristics
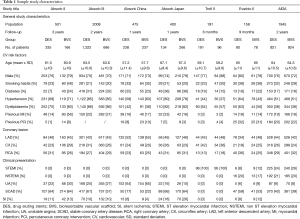
Population characteristics for each studies are listed in Table 1. The whole study population was of 5,674 patients. The mean age of the population was 62.2±1.31 in the DES group and 62±1.47 in the BVS group (P=0.942). Overall, males were 74%, 26% were smokers, 24% had diabetes and finally hypertension and dyslipidaemia were present in 64% of the study population. The majority of patients enrolled had a diagnosis of stable coronary artery disease (48%) or of unstable angina (21%). Left anterior descendent artery was the most treated vessel (51%).
Full table
Outcomes
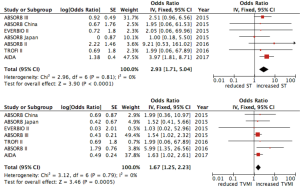
The rate of DOCE was 7% for patients treated with DES and 9% in the group treated with BVS (OR 1.16, 95% CI: 0.90–1.48; P=0.259, I2=0%) (Figure 2). The occurrence of cardiac death was similar between patients treated with both DES and BVS (OR 0.86, 95% CI: 0.52–1.40; P=0.537, I2=0%) (Figure 2) while ischemia-driven target lesion revascularization was more incident in the BVS group (OR 1.32, 95% CI: 1.01–1.73; P=0.039, I2=0%) (Figure 3).
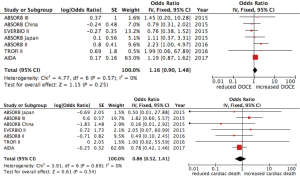
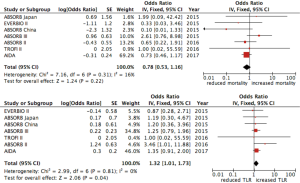
No difference was found in all-cause mortality between the two groups (OR 0.78, 95% CI: 0.53–1.15; P=0.205, I2=15%) (Figure 3). Device thrombosis was more frequent in patient treated with BVS (2.2% vs. 0.6%, OR 2.94, 95% CI: 1.71–5.05, P=0.0001, I2=0%) as well as target-vessel MI (5.4% vs. 3%, OR 1.66, 95% CI: 1.25–2.21, P=0.001, I2=0%) (Figure 4).
Meta-regression and subgroup analysis
Meta-regression analysis showed that no-one of clinical patients’ characteristics affected the outcomes considered. After stratification of study according the length of the follow-up (≤1 vs. >1 year) the results for all the outcomes considered did not change.
Publication bias
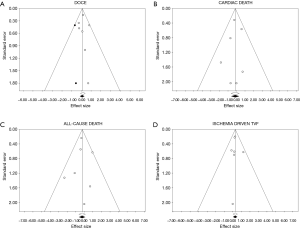
Tests for the detection of publication bias were negative for all the outcomes considered (Table 2) (Figures 5-7).
Full table

Discussion
The present meta-analysis valued BVS versus DES selecting only RCT. Data show that there is not any advantage between BVS and DES implantation in terms of mortality, cardiac death, and DOCE. However, the rate of ID-TLR, device thrombosis and of target vessel MI is higher in the group of patients treated with BVS. This confirm the trend of the single big trials (ABSORB III and AIDA), recently published and which failed to demonstrate a safety profile of these scaffolds (25,26). Meta-regression analysis showed that the main clinical characteristics of the patients are not influencing the outcomes analysed. Moreover, the heterogeneity was insignificant in all the analyses, showing a good reliability of the data obtained.
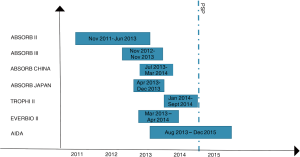
The implantation technique of BVS is different from the one used for common DES. In the last years, a European expert consensus (GHOST-EU registry) has proposed a specific protocol to be followed for the correct apposition of scaffolds, the so called “PSP technique” (27). This technique consists in preparation of the lesion, with Pre-dilatation, accurate vessel Sizing (28,29), and mandatory post-dilatation (PSP) (30). Unfortunately, all data coming from big trials are on patients treated with BVS, enrolled between November 2011 and December 2015 (Figure 8). The PSP technique was firstly reported in the late 2014, so only few data coming from trials reflect the correct technique for BVS implantation. Moreover, a recent study on GHOST-registry population demonstrated that a specific model of PSP translated in a score, was an independent predictor of DOCE, and that patients with high PSP score had less events, particularly MI (31). The limit of this technique are several; first of all, costs: the correct sizing requires the use of intracoronary imaging; secondly the time for the procedure will increase. Thirdly, there are no data coming from RCT. In the AIDA trials, in 26 over 31 stent thrombosis occurred in the BVS group, the post dilatation was performed, but with only 16 ATM (25). In addition, only in ten cases the post-dilatation was performed with a ballon ≥0.5 mm the diameter of the scaffold (25). New data showed best results with a post dilation at 18–20 ATM (32). Hence, if with PSP the BVS implantation will became a safety technique it still has to be proven. Nevertheless, several registries showed that a scrupulous implantation technique is associated with a better outcome, similar to that observed with second generation DES, also in complex clinical scenario (33). As a matter of fact, the mechanical structure of second generation DES, ensures a good result of stent deployment even if post-dilatation results in under-expansion. This is not equally true in the setting of scaffold, where a careful implantation with some tips and tricks could significantly change the long-term performance. It is important to underline that: (I) the scaffold struts are thicker and post-dilation helps in the correct deliverability process; (II) scaffolds have not radial strength, because of the absence of a metallic structure; (III) the delivery balloon used for BVS is usually more compliant than the ones used with other DES. For these and other under-investigation reasons, a proper sizing followed by post-dilatation should be considered mandatory and the absence of compliance with this behaviour might justify the clinical failure in the first RCTs involving scaffolds.
In the first three months after BVS implantation there is mainly the delivery of the drug; in the first year the mechanical support of the scaffold disappears, but the full mass loss and reabsorption of the scaffold require up to three years (34). The physiological process of scaffold dissolution may be related to an increased thrombotic risk compared to DES design, and this could be overpassed with stronger or prolonged regimen of antiplatelet therapy until the complete reabsorption of the scaffold (35). Finally, all the data we analysed where on a follow-up of maximum three years, where, the real advantage of the design of scaffolds are related to the complete restoration of the physiology of vessel wall (34), which happens in three years, but if there is an advantage in the full restoration of the vessel wall, only a longer follow-up will be able to verify the benefit of BVS implantation versus DES.
New trials are needed to explore these three hypothesis and to definitely understand if there is any advantage in BVS implantation. In particular, ABSORB IV (36) will answer some of this question.
Study limitation
This is a study level meta-analysis. We calculated the OR using single event data from each paper considered, so data obtained are not weighted for other factors even if meta-regression analysis was negative for the main clinical characteristics of the study population.
Conclusions
The implantation of BVS is related to an increased risk of device thrombosis, ischemia-driven target lesion revascularization and target vessel MI, while the rate of DOCE, cardiac death and all-cause death do not differ for patients treated with BVS or DES. New trials valuing if a different implantation technique may change these findings are clearly on demand. Similarly, longer follow-up is needed to analyse the benefit of BVS implantation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Palmerini T, Biondi-Zoccai G, Stone GW. Stent selection to minimize the risk of stent thrombosis. Curr Opin Cardiol 2014;29:578-85. [Crossref] [PubMed]
- Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis - Strut coverage as a marker of endothelialization. Circulation 2007;115:2435-41. [Crossref] [PubMed]
- Finn AV, Nakazawa G, Joner M, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol 2007;27:1500-10. [Crossref] [PubMed]
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193-202. [Crossref] [PubMed]
- Hofma SH, Van Der Giessen WJ, Van Dalen BM, et al. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J 2006;27:166-70. [Crossref] [PubMed]
- Togni M, Windecker S, Cocchia R, et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol 2005;46:231-6. [Crossref] [PubMed]
- Onuma Y, Serruys PW. Bioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011;123:779-97. [Crossref] [PubMed]
- Bourantas CV, Zhang Y, Farooq V, et al. Bioresorbable scaffolds: current evidence and ongoing clinical trials. Curr Cardiol Rep 2012;14:626-34. [Crossref] [PubMed]
- Zhang Y, Bourantas CV, Farooq V, et al. Bioresorbable scaffolds in the treatment of coronary artery disease. Med Devices (Auckl) 2013;6:37-48. [PubMed]
- Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM Statement. Rev Esp Salud Publica 2000;74:107-18. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology - A proposal for reporting. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2009. Available online: http://handbook.cochrane.org. Accessed 13 October 2016.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Pinto Slottow TL, Waksman R. Overview of the 2006 food and drug administration circulatory system devices panel meeting on drug-eluting stent thrombosis. Catheter Cardiovasc Interv 2007;69:1064-74. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Cooper H, Hedges VL, Valentine JC. Handbook of Research Synthesis and Meta-Analysis. Second Edition. 2009.
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479-91. [Crossref] [PubMed]
- Nathan A, Kobayashi T, Kolansky DM, et al. Bioresorbable scaffolds for coronary artery disease. Curr Cardiol Rep 2017;19:5. [Crossref] [PubMed]
- Kimura T, Kozuma K, Tanabe K, et al. A randomized trial evaluating everolimus-eluting Absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J 2015;36:3332-42. [Crossref] [PubMed]
- Puricel S, Arroyo D, Corpataux N, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol 2015;65:791-801. [Crossref] [PubMed]
- Sabaté M, Windecker S, Iñiguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J 2016;37:229-40. [Crossref] [PubMed]
- Gao R, Yang Y, Han Y, et al. Bioresorbable vascular scaffolds versus metallic stents in patients with coronary artery disease: ABSORB China trial. J Am Coll Cardiol 2015;66:2298-309. [Crossref] [PubMed]
- Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med 2017;376:2319-2328. [Crossref] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT01751906
- Tamburino C, Latib A, Van Geuns RJ, et al. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: a European perspective. EuroIntervention 2015;11:45-52. [Crossref] [PubMed]
- Biscaglia S, Secco GG, Tumscitz C, et al. Optical coherence tomography evaluation of overlapping everolimus-eluting bioresorbable vascular scaffold implantation guided by enhanced stent visualization system. Int J Cardiol 2015;182:1-3. [Crossref] [PubMed]
- Biscaglia S, Campo G, Tebaldi M, et al. Bioresorbable vascular scaffold overlap evaluation with optical coherence tomography after implantation with or without enhanced stent visualization system (WOLFIE study): a two-centre prospective comparison. Int J Cardiovasc Imaging 2016;32:211-23. [Crossref] [PubMed]
- Imori Y, D'ascenzo F, Gori T, et al. Impact of postdilatation on performance of bioresorbable vascular scaffolds in patients with acute coronary syndrome compared with everolimus-eluting stents: A propensity score-matched analysis from a multicenter "real-world" registry. Cardiol J 2016;23:374-83. [Crossref] [PubMed]
- Ortega-Paz L, Capodanno D, Gori T, et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events: development and internal validation of the PSP score. EuroIntervention 2017;12:2110-7. [Crossref] [PubMed]
- De Ribamar Costa J, Abizaid A, Bartorelli AL, et al. Impact of post-dilation on the acute and one-year clinical outcomes of a large cohort of patients treated solely with the Absorb Bioresorbable Vascular Scaffold. EuroIntervention 2015;11:141-8. [Crossref] [PubMed]
- Biscaglia S, Ugo F, Ielasi A, et al. Bioresorbable Scaffold vs. Second Generation Drug Eluting Stent in Long Coronary Lesions requiring Overlap: A Propensity-Matched Comparison (the UNDERDOGS study). Int J Cardiol 2016;208:40-5. [Crossref] [PubMed]
- Oberhauser JP, Hossainy S, Rapoza RJ. Design principles and performance of bioresorbable polymeric vascular scaffolds. EuroIntervention 2009;5 Suppl F:F15-22.
- Capodanno D, Angiolillo DJ. Antiplatelet therapy after implantation of bioresorbable vascular scaffolds: a review of the published data, practical recommendations, and future directions. JACC Cardiovasc Interv 2017;10:425-37. [Crossref] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT02173379