What is the difference between FEV1 change in percentage predicted value and change over baseline in the assessment of bronchodilator responsiveness in patients with COPD?
Introduction
Chronic obstructive pulmonary disease (COPD), a common preventable and treatable disease, is characterized by persistent airflow obstruction that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases (1). Bronchodilator responsiveness plays an important role in the identification and treatment of COPD while combining with medical history and clinical data. In many clinical trials of COPD (2-4), patients were eligible for inclusion with poor forced expiratory volume in one second (FEV1) responsiveness only, although other lung function parameters such as forced vital capacity (FVC), inspiratory capacity (IC), peak expiratory flow, forced expiatory flow at 25% to 75%, specific airway conductance and airway resistance have been applied for the assessment of bronchodilator responsiveness in clinical practice.
The use of FEV1 (and/or FVC) percentage change and absolute change over baseline with cut-off thresholds for determining significant bronchodilator responsiveness was recommended by American Thoracic Society (ATS) and European Respiratory Society (ERS) in the statement of standardization (5) and interpretation (6) for lung function tests. This criterion based on FEV1 was adopted by Global initiative for Chronic Obstructive Lung Disease (GOLD) (1) and Global Initiative for Asthma (GINA) (7) guidelines. However, the criterion of FEV1 absolute change in percentage predicted value (ΔFEV1%pred) with a cut-off threshold also was widely applied in many studies (2-4,8,9) for assessing significant FEV1 responsiveness. Some investigators even addressed FEV1 percentage predicted criterion was more advanced in distinguishing COPD from asthma (8,10).
The difference between FEV1 change in percentage predicted value and change over baseline in the assessment of significant FEV1 responsiveness in patients with COPD was reported by Tashkin (11), Hanania (12) and Anthonisen (13). However, the difference in various degree of severity among these criteria was not revealed in detail. Is the difference related to the degree of severity of COPD? Which criterion is better for clinical practice? Meanwhile, the use of FVC as an outcome of bronchodilator responsiveness was ignored in most studies of COPD. Is there any clinical meaning of FVC responsiveness in COPD? Better understanding the difference of these criteria will impact on the diagnosis and treatment strategy of COPD. Therefore, the purpose of this study was: (I) to assess pre-defined cut-off thresholds for significant acute bronchodilator responsiveness; (II) to define the possibility of FVC as a suitable parameter assessing bronchodilator responsiveness apart from FEV1; and (III) to define the relationship between disease severity and response to bronchodilator in FEV1 as well as FVC. The distribution of the response to bronchodilator was an explorative observation in the present study.
Subjects and methods
Patients
This study retrospectively analysed 933 stable patients with COPD diagnosed by chest physicians in our hospital from January 2004 to July 2009. They were aged ≥40 years, had dyspnoea, chronic cough, and/or sputum production, and/or a history of exposure to risk factors for the disease, and had a post-bronchodilator FEV1/FVC of <0.70. Patients were excluded if they had a history of asthma or pulmonary resection, had an exacerbation of COPD or a respiratory infection within 4 weeks, used supplemental oxygen for >12 hours per day, or had significant diseases that might influence the results of the study or patient’s ability to perform spirometry. The demographics and baseline characteristics are shown in Table 1.
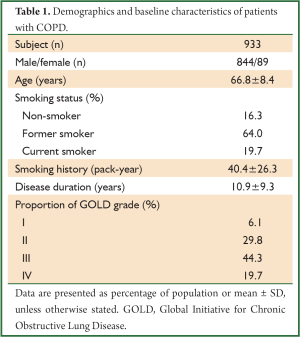
Full Table
The protocol was approved by local ethnic committee and written informed consent was obtained from all patients in the study. All information was kept confidentially.
Methods
Spirometers (Jaeger Masterscreen, Germany; Cosmed PFT Quark, Italy; Sensormedics, USA; Medisoft Body, Belgium) all met the instrument standardization of American Thoracic Society and European Respiratory Society (ATS-ERS) (5). Technicians who performed spirometry were trained and certified. Calibration check was undertaken daily prior to spirometry by a 3,000 mL syringe, and validated that the devices were within calibration limits, e.g., ±3% of true (90 mL). With correct sitting posture, patients attached nose clip or manual occlusion of the nares, placed mouthpiece in mouth and closed lips around the mouthpiece. Then they were encouraged to inhale completely and rapidly with a pause of <1 s at total lung capacity, and exhale maximally until no more air can be expelled while maintaining an upright posture. Acceptable manoeuvre: (I) free from artefacts (cough during the first second of exhalation; glottis closure that influenced the measurement; early termination or cut-off; effort that was not maximal throughout; leak or obstructed mouthpiece); (II) good starts (extrapolated volume less than 5% of FVC or 0.15 L, whichever is greater); (III) satisfactory exhalation (a plateau in the volume-time curve or forced expiratory time of >6 s or if the subject cannot or should not continue to exhale). Manoeuvers were repeated for 3 to 8 times in each test until three acceptable and repeatable spirograms were obtained. For both FVC and FEV1, the acceptable difference was within 0.15 L of the largest and second largest values. Then the largest FVC and FEV1 were reported. Predicted values of FVC and FEV1 were selected from the statement of European Committee of Steel and Coal (14) and adjusted for Chinese with the recommendation of Zheng and Zhong (15). Technique for performing spirometry that met quality criteria according to the standardisation (5) and interpretation (6) of ATS-ERS has been published elsewhere (16-18).
Prior to bronchodilation test, medication wash-out requirements included withholding short- and long-acting β-agonists (for ≥6 and ≥12 hours, respectively), short- and long-acting anticholinergic agent (for ≥6 and ≥24 hours, respectively), short- and long-acting theophylline (for ≥24 and ≥48 hours, respectively) and anti-leukotrienes (for ≥48 hours). Patients were not permitted to smoke, exercise or have a tea/coffee within 6 hours before spirometry. Spirometry was performed before and 20 to 30 minutes after 400 micrograms of salbutamol administered via spacer by metered dose inhaler (MDI). The change of FEV1 was expressed as: (I) FEV1 percentage change over baseline (ΔFEV1%); (II) absolute change in percentage predicted value (ΔFEV1%pred); (III) absolute change over baseline (ΔFEV1). The change of FVC was expressed similarly as FEV1. The significant bronchodilator responsiveness was assessed by the following criteria: (I) ATS-ERS criterion based on FEV1: ΔFEV1% ≥12% and ΔFEV1 ≥200 mL, (II) FEV1 percentage predicted criterion: ΔFEV1%pred ≥10%, (III) ATS-ERS criterion based on FVC: FVC percentage change over baseline (ΔFVC%) ≥12% and FVC absolute change over baseline (ΔFVC) ≥200 mL. The grade (degree of severity) of COPD was defined by GOLD guideline (1).
These data were analysed by SPSS software 15.0 (Chicago, IL, USA). The demographics and baseline characteristics were presented as percentage of population or mean ± SD. After bronchodilator inhalation, the variation and distribution of FEV1 and FVC changes were described. Significant FEV1 responsiveness of COPD in different degree of severity was assessed using ATS-ERS criterion and FEV1 percentage predicted criterion, and the differences between these criteria were examined with McNemar Test. Logistic regression with stepwise selection procedure for significant bronchodilator responsiveness was performed among these criteria. P<0.05 was considered as statistically significant.
Results
Pre- and post-bronchodilator spirometry in patients with COPD
Of the patients, pre-bronchodilator FEV1 and FVC were 974±448 and 2,242±703 mL, respectively. Mean improvements after bronchodilator inhalation were 122 mL in FEV1 and 264 mL in FVC (both versus baseline, P<0.001). The changes of FEV1 and FVC in different grade of COPD are shown in Table 2. As the degree of severity increased, the mean improvement of FEV1 was reduced; on the contrary, that of FVC was increased.

Full Table
Variation and distribution of FEV1 change after bronchodilator inhalation
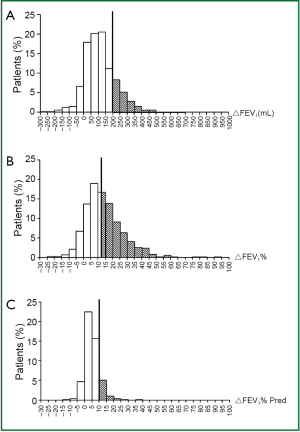
Improvement of FEV1 was found in 856 out of 933 patients (91.7%). Of all the patients, 13.5% met ΔFEV1%pred ≥10%, 22.3% met ΔFEV1 ≥200 mL and 49.8% met ΔFEV1% ≥12%. When ATS-ERS criterion based on FEV1 and FEV1 percentage predicted criterion were evaluated independently, the percentage of patients considered to show significant responsiveness differed substantially (21.4% versus 13.5%, χ2=59.5, P<0.001). The variation and distribution of FEV1 change (including ΔFEV1, ΔFEV1% and ΔFEV1% pred) are shown in Figure 1.
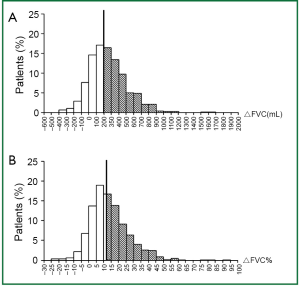
Variation and distribution of FVC change after bronchodilator inhalation
Improvement of FVC was found in 819 out of 933 patients (87.8%). Of all the patients, 56.0% met ΔFVC ≥200 mL and 46.7% met ΔFVC% ≥12%. When ATS-ERS criterion based on FVC and ATS-ERS criterion based on FEV1 were evaluated independently, the percentage of patients considered to show significant responsiveness differed substantially (45.3% versus 21.4%, χ2=154.07, P<0.001). The variation and distribution of FVC change (including ΔFVC and ΔFVC%) are shown in Figure 2. Of all the patients, 50.7% met ATS-ERS criterion based on FEV1 or/and FVC.
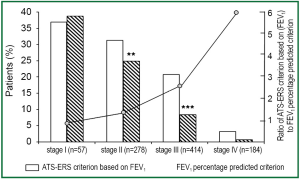
Significant bronchodilator responsiveness in different grades of COPD
The percentages of patients with COPD met ATS-ERS criterion based on FEV1 and FEV1 percentage predicted criterion were 36.8% and 38.6% in grade I (P=1.00), 31.3% and 24.8% in grade II (P<0.01), 20.8% and 8.2% in grade III (P<0.001), 3.24% and 0.54% in grade IV (P=0.074), respectively (Figure 3). The responsive ratios of ATS-ERS criterion based on FEV1 to FEV1 percentage predicted criterion were 0.95 in grade I, 1.26 in grade II, 2.53 in grade III and 6.00 in grade IV, respectively.
Using ATS-ERS criterion based on FVC, significant responsiveness were found in 26.3% of grade I, 36.7% of grade II, 48.8% of grade III and 56.5% of grade IV, respectively. Furthermore, 40.4% of grade I, 47.1% of grade II, 51.9% of grade III and 56.5% of grade IV met ATS-ERS criterion based on FEV1 or/and FVC, respectively.
Logistic regression with stepwise selection procedure for significant bronchodilator responsiveness
The significant variables from the multivariate logistic regression model are demonstrated in Table 3. The odds of milder COPD patient tended to be higher for meeting the criterion based on FEV1 (P<0.001), whereas lower odds for meeting ATS-ERS criterion based on FVC (P<0.001). The model also showed that higher odds of significant responsiveness were associated with shorter disease duration by ATS-ERS criterion based on FEV1 (P=0.011) or FEV1 percentage predicted criterion (P=0.008); on the contrary, lower odds of significant responsiveness were associated with shorter disease duration by ATS-ERS criterion based on FVC (P=0.02). Predicted value of FEV1 was a significant factor only for ATS-ERS criterion based on FEV1 and a patient with higher predicted value of FEV1 tended to exhibit higher odds of being responsive under this criterion (P<0.001). However, age, gender, body mass index, smoking status, smoking history, predicted value of FVC did not show significance in the logistic regression in all cases.
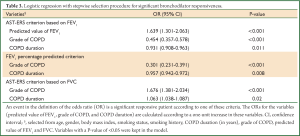
Full Table
Discussion
Up to 22.4% of patients met at least one criterion for significant FEV1 responsiveness after bronchodilator inhalation in the present study. However, when ATS-ERS criterion based on FEV1 and FEV1 percentage predicted criterion were evaluated independently, the percentage of patients considered to show significant responsiveness differed substantially (21.4% versus 13.5%). This was consistent with the findings of Tashkin (11) and Hanania (12), although the percentages of patients with significant responsiveness were lower than that of their reports, probably due to more bronchodilator inhalation (80 micrograms of ipratropium followed by 400 micrograms of salbutamol) and less severe patients compared with the present study.
To our knowledge, bronchodilator responsiveness among different criteria in various degree of severity was not addressed at length in patients with COPD. In the present study, there was statistical significant difference between ATS-ERS criterion based on FEV1 and FEV1 percentage predicted criterion with significant responsiveness only in grade II and III of COPD. However, if we pay attention to the responsive ratios of these criteria, an obvious trend could be found across all the grades of COPD, indicating the more severity in airflow obstruction, the larger difference between the two criteria. None significant difference in grade I of COPD was probably due to the insufficient number of mild patients, while in grade IV, due to the very poor FEV1 responsiveness. As reported by Zhong (16) in a large, population-based survey, the prevalence of COPD in China was 8.2% of people aged ≥40 years and the distributions of grade I, II, III, and IV of COPD were 25.3%, 48.1%, 21.5% and 5.1%, respectively. That indicated moderate-to-severe patients were accounted for over 2/3 of the large COPD population. Therefore, the significant differences between these criteria in grade II and III of COPD are very important and could impact on the diagnosis and treatment strategy of COPD.
Patients with milder grade or shorter disease duration of COPD appeared to more often meet both criteria based on FEV1. Probably due to the insufficient number of female, gender didn’t show significance in the logistic regression of significant bronchodilator responsiveness regardless of whichever criterion was applied, but a patient with higher predicted value of FEV1 tended to exhibit higher odds for meeting ATS-ERS criterion based on FEV1 (e.g., female has smaller lung volumes, comparing with male). However, self-reported cigarette use (pack-year) wasn’t associated with significant bronchodilator responsiveness regardless of whichever criterion was applied, which differed from Tashkin’s report (11). Therefore, the value of self-reported disease duration and smoking history should be viewed with caution.
The present study demonstrated that 422 of 931 patients had significant responsiveness with ATS-ERS criterion based on FVC, which was similar with Ben Saad H’s (77 of 168 patients) and Walker PP’s (125 of 266 patients) reports (19,20). However, their studies did not address the responsiveness after bronchodilator in various degree of severity. In our study, as the degree of severity of COPD increased, the improvement of FEV1 was reduced; on the contrary, that of FVC was increased, and more patients met ATS-ERS criterion based on FVC. Interpretation strategies for lung function tests of ATS-ERS addressed that significant improvement in the FEV1, FVC or both would suggest the presence of reversible airflow obstruction (6), which is the ability to achieve a certain threshold of bronchodilator responsiveness.
As we know, FEV1 here represents flow response in particular, while FVC represents the volumetric response to bronchodilator. In fact, after bronchodilator treatment in the clinical practice, symptoms of dyspnoea (such as BDI/TDI) and exercise tolerance (such as 6-minutes’ walk distance) in some patients with COPD improved a lot, especially in more severity of airway obstruction. This phenomenon could not be explained by the airflow improvement due to no significant increase in FEV1. On the other hand, this can be explained by the improvement of FVC, representing airway opening, reduction of air trapping or reduction of residual volume. Of cause, the role of FVC change as an index of acute responsiveness of airway obstruction in COPD to be used for therapeutic purposes should be studied in the future. FVC might not be a good index to distinguish asthma from COPD, but further studies to confirm the hypothesis that FVC is a better index than FEV1 in assessing the treatment response by the correlation between prognosis parameters (such as quality of life, exacerbation or 6 minutes’ walk distance, etc.) and change of FEV1 as well as FVC is optimized. Unfortunately, only FEV1 has been focused on in most studies of COPD.”
In addition, some investigators used IC to assess bronchodilator responsiveness of COPD (21,22). The same meaning as FVC, increase of IC after bronchodilator inhalation suggests a reduction of dynamic hyperinflation. However, FVC could be obtained simultaneously when spirometry manoeuvre was performed; by comparison, IC has to be tested separately. Moreover, the reliability and quality control of IC are poor in some instance. Therefore, FVC have the advantage of easier obtainment in the clinical practice.
Compared with ATS-ERS criterion based on FEV1, FEV1 percentage predicted criterion identified higher percentage of patients without significant responsiveness at every spirometry clinic visit in the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial (12). Our finding showed that FEV1 percentage predicted criterion (FEV1 responsiveness only) underestimated the treatment response of patients with COPD, while better significant responsiveness was revealed by ATS-ERS criterion based on FEV1 and FVC (the later in particular), which not only encouraged people to change pessimism into optimism in the treatment, but also better reflected the true bronchodilator responsiveness of COPD (including both FEV1 responsiveness and FVC responsiveness), especially for more severity of airway obstruction. In fact, most patients who visited hospitals or clinics frequently were in severe condition. Consequently, we preferred ATS-ERS criterion based on FEV1 and FVC to FEV1 percentage predicted criterion in clinical practice.
There were some limitations in our study. The definition of airflow obstruction by the fixed FEV1/FVC of 0.70 could lead to over-diagnosis of COPD in elder population (23). Use of lower-limit-of-normal (LLN) of FEV1/FVC might be a better choice, but we selected 0.70 as the cut-off point due to the lack of LLN in Chinese population at the moment, and this cut-off point is more practical and recommended by many international guidelines to identify subjects with COPD (1). The present study didn’t record the data of daily medications used for COPD, which might influence the result of lung function test, but that was not a major factor to affect our conclusion because patients were required to withdraw bronchodilator or other drugs before spirometry and only a small proportion of Chinese patients took the treatment for COPD [e.g., 22.7% of total patients used any medicine for COPD in our previous study (3)]. In addition, mild patients and female patients were relatively insufficient, although it did reflect the real life of COPD management in China. Nevertheless, the present study still found the evidence of difference among these criteria and the different improvement between FEV1 and FVC. Finally, the trend of bronchodilator responsiveness over time was not studied in the present research. These limitations will be concerned in future studies.
In conclusion, compared with FEV1 percentage predicted criterion, ATS-ERS criterion based on FEV1 as well as FVC, the later in particular, detected a larger percentage of patients with significant responsiveness. The increasing difference was relevant as a function of the severity of airflow obstruction.
Acknowledgements
We thank Tian’en Jin*, Pingping Guo*, Mengjie Jiang* and Bo Yun* for collection of some data. (*Guangzhou Medical University, Guangzhou, China.)
Disclosure: Supported by Development Plan of ChangJiang Scholars and Innovative Research Team (ITR0961), and The National Key Technology R&D Program of the 12th National Five-year Development Plan (2012BAI05B01).
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD. Available online: http://www.goldcopd.org/ (Revised 2013).
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89. [PubMed]
- Zheng JP, Yang L, Wu YM, et al. The efficacy and safety of combination salmeterol (50 microg)/fluticasone propionate (500 microg) inhalation twice daily via accuhaler in Chinese patients with COPD. Chest 2007;132:1756-63. [PubMed]
- Barnes NC, Qiu YS, Pavord ID, et al. Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med 2006;173:736-43. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [PubMed]
- Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008;31:143-78. [PubMed]
- Silvestri IC, Pereira CA, Rodrigues SC. Comparison of spirometric changes in the response to bronchodilators of patients with asthma or chronic obstructive pulmonary disease. J Bras Pneumol 2008;34:675-82. [PubMed]
- Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008;177:19-26. [PubMed]
- Brand PL, Quanjer PH, Postma DS, et al. Interpretation of bronchodilator response in patients with obstructive airways disease. The Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group. Thorax 1992;47:429-36. [PubMed]
- Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J 2008;31:742-50. [PubMed]
- Hanania NA, Sharafkhaneh A, Celli B, et al. Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respir Res 2011;12:6. [PubMed]
- Anthonisen NR, Lindgren PG, Tashkin DP, et al. Bronchodilator response in the lung health study over 11 yrs. Eur Respir J 2005;26:45-51. [PubMed]
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5-40. [PubMed]
- Zheng J, Zhong N. Normative values of pulmonary function testing in Chinese adults. Chin Med J (Engl) 2002;115:50-4. [PubMed]
- Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007;176:753-60. [PubMed]
- Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis 2008;12:703-8. [PubMed]
- Zheng JP, Kang J, Huang SG, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet 2008;371:2013-8. [PubMed]
- Ben Saad H, Préfaut C, Tabka Z, et al. The forgotten message from gold: FVC is a primary clinical outcome measure of bronchodilator reversibility in COPD. Pulm Pharmacol Ther 2008;21:767-73. [PubMed]
- Walker PP, Calverley PM. The volumetric response to bronchodilators in stable chronic obstructive pulmonary disease. COPD 2008;5:147-52. [PubMed]
- Tonnel AB, Tillie-Leblond I, Attali V, et al. Predictive factors for evaluation of response to fluticasone propionate/salmeterol combination in severe COPD. Respir Med 2011;105:250-8. [PubMed]
- Celli BR, Tashkin DP, Rennard SI, et al. Bronchodilator responsiveness and onset of effect with budesonide/formoterol pMDI in COPD. Respir Med 2011;105:1176-88. [PubMed]
- Johannessen A, Lehmann S, Omenaas ER, et al. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med 2006;173:1316-25. [PubMed]


