The missed diagnosis of aortic dissection in patients with acute myocardial infarction: a disastrous event
Introduction
The morbidity rate of acute aortic dissection (AD) is 6 cases per 100,000 person-years (1). Approximately 7% of patients with the AD have concomitant acute myocardial infarction (AMI) (2). Nevertheless, in patients with AMI secondary to AD, the omission diagnostic rate of AD has been as high as 30% (3). To attenuate the omission diagnostic rate of AD, we reported one case to recommend that the portable echocardiography should be routinely available in the ambulance.
Case presentation
A previously healthy 40-year-old man with sudden chest tightness and dull pain in the chest of 2-hour duration, in the absence of tearing pain radiating to the thorax or the back was rushed to our hospital in an ambulance. During this period, the patient became unconscious for 2 minutes. He had been smoking (20 cigarettes/day) for 15 years and drinking (200 g/day) for 25 years. He had no history of hypertension, diabetes, or a family history of cardiovascular diseases. Physical examinations showed the patient had a body temperature of 37 °C, a pulse of 97 bpm, a respiratory rate of 26 bpm, blood pressure in left arm of 65/50 mmHg and in right arm of 63/48 mmHg, fingertip oxygen saturation of 97%, height of 178 cm, and weight of 65 kg. The patient was in poor condition, with a pinched face, but was conscious and cooperative for the vital statistics check. His breath sounds were clear without rhonchus and crackles. His heart rate was 97 bpm, with a powerful heart sound and regular heart rhythm without murmurs among the auscultatory valve areas. His abdomen was soft without pain on compression and without vascular murmurs. The electrocardiogram (ECG) demonstrated ST-segment elevation in leads aVR, V1–V3, T-wave inversion in lead III, and ST-segment depression in leads I, II, aVL, aVF, and V4–V6 (Figure 1). Laboratory tests were as follows: white blood cell (WBC), 13.31×109/L; granulocyte proportion, 86.00%; cardiac troponin I (cTnI), 0.13 ng/mL; creatine kinase MB (CK-MB), 2.8 ng/mL; myoglobin (MYO), >500 ng/mL; and D2 dimer, 5.50 mg/L.
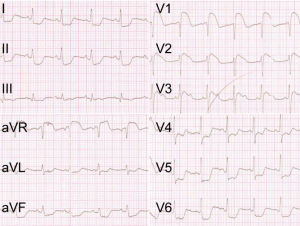
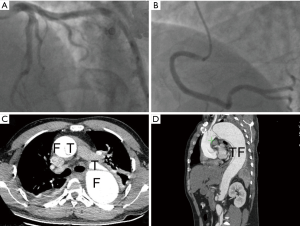
The sudden chest tightness and chest pain, ST-segment elevation in leads aVR and V1–V3, as well as elevated MYO and cTnI levels, strongly supported the diagnosis of acute anteroseptal myocardial infarction (4). ST-segment elevation in lead aVR and hypotension suggested the possible occlusion of the left main coronary artery (LM). Therefore, the patient was immediately given aspirin 300 mg and ticagrelor 180 mg orally in the ambulance, and afterward was sent directly to the cardiac catheterization laboratory for the primary coronary angiography (CAG), after being administered a heparin bolus of 3,000 U intravenously. The CAG showed no stenosis in the LM, the left anterior descending coronary artery (LAD), the left circumflex coronary artery (LCX), or the right coronary artery (RCA) (Figure 2A,B). However, the patient continued to have chest pain and hypotension. Considering the possibility of pulmonary embolism (PE), the patient was immediately given an emergency computed tomography angiography (CTA) of the pulmonary artery. The CTA ruled out PE, but displayed the existence of AD (DeBakey I): the ascending aorta, the aortic arch, and the descending aorta were all saturated with the tearing tunica intima, and the false lumen was much larger than the true lumen (Figure 2C,D). Thus, the patient was transferred into the department of cardiac surgery for operation. It was observed during the surgery that the ascending aorta, the aortic arch, the innominate artery, as well as the left common carotid artery, all formed the dissection together with the detachment of the RCA, and the false lumen was much larger than the true lumen. The patient received several surgical procedures, including the Bentall procedure, total aortic arch replacement combined with stented elephant trunk implantation, and coronary artery bypass grafting (CABG). He recovered well post operation and was discharged from the hospital.
Discussion
Approximately 7% of patients with the AD have concomitant AMI. Nevertheless, in patients with AMI secondary to AD, the omission diagnostic rate of AD has been as high as 30%. This may be because the diagnosis of AMI depends mainly on clinical manifestations, the ECG, and cTnT or cTnI level, whereas the AD can only be confirmed by imaging modalities (i.e., it is hardly possible to diagnose AD at the time of first medical contact). In particular, the guideline for patients with ST-segment elevation myocardial infarction (STEMI) is that antiplatelet therapy should be given at the time of first medical contact, and patients should be sent to the cardiac catheterization laboratory for the interventional diagnosis and treatment as soon as possible (5). These drive clinicians to pay more attention to the AMI, and thereby they tend to neglect the diagnosis of AD. However, for patients with AMI, the missed diagnosis of AD could be catastrophic, because the antiplatelet therapy and the cardiac catheterization, which are the therapeutic approaches for AMI, are exactly two absolute contraindications to AD treatment, as both of them can aggravate bleeding, broaden the range of the dissection, and even increase the risk of death.
In practice, the definite diagnosis of AD relies solely on imaging examinations, including echocardiography and CTA. Among these techniques, the transthoracic echocardiography demonstrates a sensitivity of 77% to 80% and a specificity of 93% to 96% for identification of proximal AD (2). Moreover, if the risk score system of AD (2) is fully utilized simultaneously, the sensitivity of the echocardiography may be further increased. Importantly, the transthoracic echocardiography is portable and can be conveniently equipped in an ambulance. Therefore, to attenuate the missed diagnosis of AD in patients with AMI, we strongly recommend that portable echocardiography should be routinely available in the ambulance and, accordingly, emergency physicians should receive necessary training to be able to perform the echocardiography. Moreover, we propose that all the patients with acute chest pain should receive an ECG and echocardiography at the time of first medical contact. When AD complicated with AMI is preliminarily ruled out, clinicians then treat patients with aspirin and P2Y12 inhibitors, and then implement the CAG. This will decrease the omission diagnostic rate of AD without delaying the antithrombotic therapy and revascularization for patients with AMI. However, to date, potent evidence-based proof showing the necessity of equipping the portable echocardiography in the ambulance is still missing. Therefore, multicenter studies concerning the cost–benefit ratio of the portable echocardiography routinely available in the ambulance should urgently be considered.
Acknowledgements
Funding: This work was supported by 81470471 from the Nature Science Foundation of China (NSFC).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031-7. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection. Eur Heart J 2001;22:1642-81. [Crossref] [PubMed]
- American College of Emergency Physicians. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:485-510. [Crossref] [PubMed]
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]


