Risk factors of ventilator-associated pneumonia in pediatric intensive care unit: a systematic review and meta-analysis
Introduction
Ventilator-associated pneumonia (VAP) is defined as nosocomial pneumonia developing 48 h or more after initiation of mechanical ventilation. It is the most common hospital-associated infection (HAI) in critically ill adult patients, and is the second most common after bloodstream infection for the pediatric population (1,2). VAP accounts for about 20% of all HAI among patients in pediatric intensive care unit (PICU) and has a rate of (2.9-21.6)/1,000 ventilator days (3,4). Furthermore, some data suggested higher mortality rate for mechanically ventilated pediatric patients with VAP compared with those without VAP (5). Hospital costs and the length of ICU stay were significantly increased for pediatric patients with VAP compared with those without VAP (6).
The epidemiology, pathogenesis, and outcome of VAP are well described in adults, however, few data exist regarding VAP in pediatric patients. Because of different anatomy, physiology and underlying illnesses from adults, it is important to identify specific prevention for this population in preventing VAP. To date, there have been some researches about risk factors of VAP in PICU, however, these results indicated that risk factors were varied or contradictory. For example, Elward AM, et al. found genetic syndrome, reintubation and transport out of the PICU were independently predicted VAP (7), however, Almuneef M, et al. indicated that only prior antibiotic therapy, continuous enteral feeding, and bronchoscopy were independent predictors of VAP (8). Another conclusion was not consistent between Elward AM (7) and Roeleveld PP (9) by use of steroids.
In order to solve these uncertainties, we endeavored to accumulate the related evidence and to make a systematic and meta-analysis.
Materials and methods
Searching
A systematic literature search of the Cochrane Library, PubMed [1966-2013], OVID [1993-2013] and Web of Science [1950-2013] were conducted to identify relevant reports. Search terms were risk factors AND (VAP OR ventilator associated pneumonia OR VAP) AND (PICU OR PICUs OR PICU OR intensive care unit for children OR intensive care unit for infant). We also searched the references of the initially identified articles by hand, including relevant review papers. We did not seek for abstracts of conference proceedings. Language of publications had no limitation. No restriction on the time was set. The query was last updated on April 2013.
Selection
Two of us (Bo Liu and Song-qin Li) independently reviewed the titles and abstracts of all relevant citations and then retrieved all potentially relevant articles identified by either reviewer. Next, the following selection criteria were applied to the full manuscripts in duplicate and independently: (I) design: cohort study or case-control study; (II) population: critical patients with mechanical ventilation for 48 h or more; (III) location: PICU; and (IV) outcomes: risk factors of VAP. We excluded studies as follows: reviews, not VAP research, not risk factors research, not getting available results. Disagreements were resolved by discussion.
Data extraction
The two reviewers (Bo Liu and Song-qin Li) independently read the entire text of the retrieved reports and extracted the following data: first author, year of publication, country, study design, study population, and number of patients with or without VAP included. In addition, we collected data on the association between various risk factors and VAP.
Data analysis and statistical methods
Statistical analyses were performed using the Review Manager (RevMan version 5.1.4; http://www.ims.cochrane.org/revman/download). To quantify the risk factors of VAP, we calculated pooled odds ratios (ORs) and 95% confidence intervals (CIs) by using the Mantel-Haenszel fixed-effect or the DerSimonian-Laird random-effects models, and Z-statistic test for over effect was done, P<0.05 was considered to be statistically significant. For all analyses, the fixed-effect model was used only when there was no heterogeneity between reports; otherwise, the random-effects model was used. The heterogeneity between reports was assessed by using the I2-statistic test, and I2 <50% means heterogeneity.
Results
Selected studies
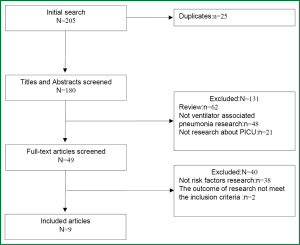
The selection process applied to identify relevant studies included in the meta-analysis is showed by a flow diagram (Figure 1). Through searching the Cochrane Library, PubMed, OVID and Web of Science, 205 potentially relevant articles were retrieved; 196 of them were excluded for the reasons detailed in Figure 1. And we did not get any relevant paper by hand-searched the references of the initially identified articles. Thus, nine studies (7-15) were included in the present meta-analysis.
Characteristics of the included studies
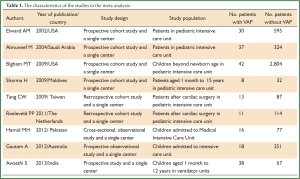
In Table 1, we list the characteristics of nine studies. The time of publication was from 2002 to 2013. Six of them were cohort study and others were case-control study. Number of patients with VAP was 213 and without VAP was 4,351.
Full Table
Factors related to VAP in studies and quality evaluation
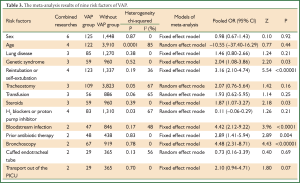
According to various risk factors including in these studies, we chose 14 risk factors in this meta-analysis. These factors included sex, age, lung disease, genetic syndrome, reintubation or self-extubation, tracheostomy, transfusion, steroids, H2 blockers or proton pump inhibitor, bloodstream infection, prior antibiotic therapy, cuffed endotracheal tube, transport out of the PICU and bronchoscopy (shown in Table 2). And we made a quality evaluation to every research by use of the Newcastle-Ottawa Scale (NOS) (16). Results of quality evaluation shown most of nine researches had high quality.

Full Table
Results of Meta-analysis about risk factors
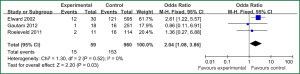
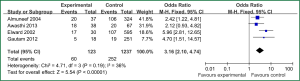
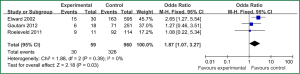
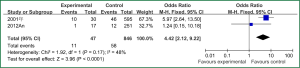
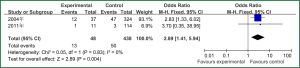
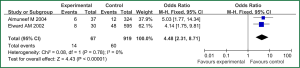
Sex of risk factors were studied in six researches; age, reintubation or self-extubation and H2 blockers or proton pump inhibitor were studied in four researches; Other risk factors were studied in less than three researches. I2 analysis for risk factors indicated that there were no heterogeneity for sex, lung disease, genetic syndrome, reintubation or self-extubation, steroids, bloodstream infection, prior antibiotic therapy, bronchoscopy and transport out of the PICU, so fixed effect model was used. However, there were heterogeneity for age, tracheostomy, transfusion, H2 blockers or proton pump inhibitor and cuffed endotracheal tube, so random effect model was used. By Z analysis, it was shown that genetic syndrome, reintubation or self-extubation, steroids, bloodstream infection, prior antibiotic therapy and bronchoscopy were risk factors for VAP (shown in Table 3). According to the meta-analysis results of these risk factors, forest plots were made respectively (Figures 2-7).
Full Table
Discussion
Understanding the pathogenesis of VAP is important for establishing the principles for therapy and strategies for prevention (17). Bacteria from aerodigestive tract above the vocal cords or stomach could be aspirated to the trachea or lung which induces VAP. In general, a complex array of host defense mechanisms protects the trachea and lungs from bacterial infection (18). However, for critically ill patients especially with mechanical ventilation, host defenses may be impaired due to malnutrition, chronic diseases or immunosuppression. One of the most important aspects of nosocomial infections is prevention. Identification of risk factors and predictors is imperative for the appropriate steps to prevent VAP.
Because of different physiology condition between children and adults, so some researches focused on risk factors of VAP in PICU. For adults, most risk factors of VAP included duration of mechanical ventilation, exposure to antibiotics, prolonged ICU stay, the presence of invasive devices, treatment with antacids or histamine type 2 receptor blockers, and advanced age (19). Despite the known consequences of VAP, unlike the situation for adults, few studies have been conducted on the epidemiology, associated risk factors, prevention, and outcomes of VAP in children (6). Although a few researches studied risk factors of VAP in PICU, the results were contradictory. So this meta-analysis tried to solve the problem.
By selection, nine researches were included in this meta-analysis. All in all, there were a lot of risk factors studied in these researches, which belonged to intrinsic or extrinsic factors. And many factors were only studied in one research. So it is necessary to reduce the number of factors. Therefore, factors that were studied in at least two researches were finally included in this meta-analysis. At last, risk factors of sex, age, lung disease, genetic syndrome, reintubation or self-extubation, tracheostomy, transfusion, steroids, H2 blockers or proton pump inhibitor, bloodstream infection, prior antibiotic therapy, cuffed endotracheal tube, transport out of the PICU and bronchoscopy were chosen. The results of meta-analysis indicated that genetic syndrome, reintubation or self-extubation, steroids, bloodstream infection, prior antibiotic therapy and bronchoscopy were risk factors for VAP of patients in PICU.
The factors of steroids and prior antibiotic therapy were similar between children and adults, which were both risk factors for VAP. The results about genetic syndrome showed that incidence of VAP for children with genetic syndrome was 2.04-fold than children without genetic syndrome. Genetic syndrome as a intrinsic factor may be a marker for comorbid conditions that might make a child more likely to be infection. For example, children with genetic syndrome have a higher PRISM score at admission, a greater number of exposure to invasive devices, and a longer ICU stay with increased opportunity for colonization and infection. Many children with genetic syndromes are associated with neuromuscular weakness, which may increase the risk of aspiration or tracheostomy (7). The results indicated that reintubation or self-extubation might be a risk factor for VAP. The most likely mechanism of this is aspiration of oropharyngeal secretions or gastrointestinal contents during the procedure. Aspiration appears to be important in the pathogenesis of VAP, as demonstrated in some studies (20,21). Patients in PICU had poor immunity because of severe illness or kinds of invasive procedure and it easily happened to be in immunodepression for them if steroids were used. Interestingly, we found H2 blockers or proton pump inhibitor was not risk factors for VAP, which argued that acid suppression may predispose to bacterial colonialization of the upper gastrointestinal tract, thus increasing VAP risk (22). Other risk factors such as the presence of invasive devices, elevating the head of bed at 30-45° are important for adults, however, it dose not fit patients in PICU. All in all, for patients in PICU, reintubation or self-extubation, steroids, bloodstream infection, prior antibiotic therapy and bronchoscopy could be preventive.
The diagnosis of VAP, however, is challenging and there is no gold standard which may explain the differences in the diverse studies concerning the occurrence of this event as well as possible risk factors (23). The researches included in this meta-analysis were published between 2002 and 2013, and from developed or developing countries, so the diagnosis used are not accordant. Therefore, the final VAP or not VAP groups in researches might affect the real outcomes.
Although there have been some researches focusing on VAP in PICU, especially on risk factors, most of them care too much factors which are difficult to get definite conclusion by systematic review. So some limitations exist in our meta-analysis, but this paper might be the first try to analyze risk factors of VAP for patients in PICU by meta-analysis. At present, little evidence is available on VAP prevention in patients in PICU, and no official guidelines have been published (24). More researches will be welcomed in the future, in order to provide more comprehensive of epidemiology, risk factors and preventions for patients in PICU.
Conclusions
This paper focused on risk factors of VAP for patients in PICU by making a meta-analysis and at last nine researches were included. According to the results, genetic syndrome, reintubation or self-extubation, steroids, bloodstream infection, prior antibiotic therapy and bronchoscopy were regarded as risk factors for VAP of patients in PICU. Special preventions should be taken for these risk factors in order to decrease the incidence of VAP. Meanwhile, further researches about risk factors and diagnosing VAP in PICU might be needed in the future.
Acknowledgments
Funding: This work was supported by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) [grant number JX10231801]; Jiangsu Province Projects of Preventive Medicine Research [grant number Y2012046].
Disclosure: The authors declare no conflict of interest.
References
- Grohskopf LA, Sinkowitz-Cochran RL, Garrett DO, et al. A national point-prevalence survey of pediatric intensive care unit-acquired infections in the United States. J Pediatr 2002;140:432-8. [PubMed]
- Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1-36. [PubMed]
- Elward AM. Pediatric ventilator-associated pneumonia. Pediatr Infect Dis J 2003;22:445-6. [PubMed]
- Tang CW, Liu PY, Huang YF, et al. Ventilator-associated pneumonia after pediatric cardiac surgery in southern Taiwan. J Microbiol Immunol Infect 2009;42:413-9. [PubMed]
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867-903. [PubMed]
- Srinivasan R, Asselin J, Gildengorin G, et al. A prospective study of ventilator-associated pneumonia in children. Pediatrics 2009;123:1108-15. [PubMed]
- Elward AM, Warren DK, Fraser VJ. Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics 2002;109:758-64. [PubMed]
- Almuneef M, Memish ZA, Balkhy HH, et al. Ventilator-associated pneumonia in a pediatric intensive care unit in Saudi Arabia: a 30-month prospective surveillance. Infect Control Hosp Epidemiol 2004;25:753-8. [PubMed]
- Roeleveld PP, Guijt D, Kuijper EJ, et al. Ventilator-associated pneumonia in children after cardiac surgery in The Netherlands. Intensive Care Med 2011;37:1656-63. [PubMed]
- Bigham MT, Amato R, Bondurrant P, et al. Ventilator-associated pneumonia in the pediatric intensive care unit: characterizing the problem and implementing a sustainable solution. J Pediatr 2009;154:582-587.e2.
- Sharma H, Singh D, Pooni P, et al. A study of profile of ventilator-associated pneumonia in children in Punjab. J Trop Pediatr 2009;55:393-5. [PubMed]
- Tang CW, Liu PY, Huang YF, et al. Ventilator-associated pneumonia after pediatric cardiac surgery in southern Taiwan. J Microbiol Immunol Infect 2009;42:413-9. [PubMed]
- Hamid MH, Malik MA, Masood J, et al. Ventilator-associated pneumonia in children. J Coll Physicians Surg Pak 2012;22:155-8. [PubMed]
- Gautam A, Ganu SS, Tegg OJ, et al. Ventilator-associated pneumonia in a tertiary paediatric intensive care unit: a 1-year prospective observational study. Crit Care Resusc 2012;14:283-9. [PubMed]
- Awasthi S, Tahazzul M, Ambast A, et al. Longer duration of mechanical ventilation was found to be associated with ventilator-associated pneumonia in children aged 1 month to 12 years in India. J Clin Epidemiol 2013;66:62-6. [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if non-randomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [PubMed]
- Grgurich PE, Hudcova J, Lei Y, et al. Management and prevention of ventilator-associated pneumonia caused by multidrug-resistant pathogens. Expert Rev Respir Med 2012;6:533-55. [PubMed]
- Craven DE, Steger KA. Epidemiology of nosocomial pneumonia. New perspectives on an old disease. Chest 1995;108:1S-16S. [PubMed]
- Elward AM, Warren DK, Fraser VJ. Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics 2002;109:758-64. [PubMed]
- Memish ZA, Oni GA, Djazmati W, et al. A randomized clinical trial to compare the effects of a heat and moisture exchanger with a heated humidifying system on the occurrence rate of ventilator-associated pneumonia. Am J Infect Control 2001;29:301-5. [PubMed]
- Marik PE, Vasu T, Hirani A, et al. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med 2010;38:2222-8. [PubMed]
- Bassetti M, Taramasso L, Giacobbe DR, et al. Management of ventilator-associated pneumonia: epidemiology, diagnosis and antimicrobial therapy. Expert Rev Anti Infect Ther 2012;10:585-96. [PubMed]
- Cooper VB, Haut C. Preventing ventilator-associated pneumonia in children: an evidence-based protocol. Crit Care Nurse 2013;33:21-9. [PubMed]