Role of cannabis in cardiovascular disorders
Introduction
Currently, cannabis is the most widely produced and consumed illicit drug in the world with global numbers of users approaching 182.5 million (3.8% of global population). Similarly, it is the most widely used illicit substance among population aged 15–64 years in North America with estimated annual prevalence of 11.6% (1). A recent longitudinal study showed that 12.4% of tobacco users reported sampling of marijuana before tobacco initiation; additionally, greater use among black youth as compared to white and among those exposed to violent trauma was also noted (2). It has been suggested that early cannabis use may be linked with higher possibility of chronic use in greater amounts.
Medical use of cannabis is widespread and dates back to many centuries. In parallel with the widespread increase in the recreational use of cannabis worldwide, especially among young adults, there have been movements towards the decriminalization of cannabis use in various parts of the world, and in some countries, cannabis prescription is now permitted for various conditions other than the traditional cancer pain and neuropathy. Recently, several countries have approved the clinical use of cannabis for various conditions. In The United States (USA), as of July 3, 2016, a total of 25 states, the District of Columbia, and Guam allow for comprehensive public medical marijuana and cannabis programs (3).
Although the widespread recreational use of cannabis is mainly due to its well-known neurological and cognitive effects, the effects of cannabis on other organ systems remain unclear to some extent and risk perceptions associated with cannabis use seem to be widely underestimated. In fact, a recent survey among a large adolescent population group in the US showed that about 3/4ths of adolescents thought that cannabis use was not associated with any significant harm (4). While the risk perception among youth is declining or remaining stable across the various states of the USA, the use of cannabis is increasing highlighting the need for public health policy changes regarding awareness of the risk to curb cannabis use among youth.
Several systematic reviews, meta-analyses, randomized control trials and case–control studies have shown that the non-medicinal use of cannabis can significantly affect physical and mental health and lead to substance dependence (5) and alter the psychosocial development and mental health of adolescents (6). The common adverse effects of cannabis use besides dependence include the risk of motor vehicle accidents, respiratory dysfunction and cardiovascular events and pathology. Cannabis use or smoking has been linked with pneumomediastinum, pneumothorax, pneumopericardium, bullous lung disease, increased risk of chronic obstructive pulmonary disease, desquamated interstitial disease, and appearance of brown pigmented macrophages (7). Jouanjus et al. (8) collected information from French Addictovigilance Network (2006–2010) and found that 1.8% of all cannabis related reports (35/1079) were cardiovascular complications, mostly men (86%) of an average age of 34.3 years. There were 22 cardiac complications (20 acute coronary syndromes), 10 peripheral vascular and 3 cerebrovascular with death rate of 26% in these patients.
Thus, it is reasonable to conclude that the growing popularity of marijuana consumption for both medical and recreational purposes is associated with a parallel increase in the incidence of complications related to its use. Therefore, there is a need for a deep understanding of the effects of marijuana on the human body. We provide a review of the endocannabinoid system (ECS) followed by examining the effects of cannabis on the cardiovascular system, including the occurrence of arrhythmias and myocardial infarction (MI) and its effects on the peripheral vasculature and the cerebrovascular system. We conducted literature search from January 1964 to October 2016 from PubMed. We also briefly examine some of the important considerations related to anaesthesia in cannabis users.
Endocannabinoid receptors in cardiovascular system
The discovery of Δ9-tetrahydrocannabinol (THC) as the main active ingredient of Cannabis sativa (9) led to the subsequent discovery of specific receptors for THC in the human body, namely cannabinoid receptors type 1 (CB-1) and type 2 (CB-2). CB-1 receptors are expressed in the liver, muscle, fat, and brain, while CB-2 receptors are expressed in large numbers in the spleen and immune cells as well as in peripheral tissues, albeit at low levels (10).These findings triggered a number of studies that eventually led to the discovery of the ECS. The ECS comprises the cannabinoid receptors (CB-1 and CB-2) as well the endogenous counterparts of THC and endogenous ligands for both cannabinoid receptors, known as N-arachidonoyl-ethanolamine (anandamide) and 2-arachidonoyl-glycerol (2-AG). The ECS has been found to be involved in a number of processes, including cell fate and proliferation and differentiation of progenitors (11). Owing to its wide representation of the ECS in the human body and its involvement in a variety of bodily processes, this system has emerged as a versatile therapeutic target. Endocannabinoids were detected in heart tissues and current evidences suggest that the ECS is involved in the regulation of heart rate (HR) and blood pressure in addition to being involved in various other pathological processes (12). Experimental studies have shown redundancy in endocannabinoid signalling and in endocannabinoid targets with dualistic role of CB-1 and CB-2 receptors in the presence of pathological conditions. Cumulative evidence seems to suggest that CB-1 and CB-2 receptors may play contributory roles in modulating cardiometabolic risk, and atherogenesis, and can also have protective roles in limiting cardiomyocyte damage (12). ECS have been found to exert vasorelaxing effects in cardiovascular system which appears to be mediated by numerous pathways. Activation of CB1 receptors in mice have been shown to produce prolonged hypotension. THC can cause vasodilatation, independent of cannabinoid receptor activation, by activating transient receptor potential ankyrin type-1 (TRPA-1) channel. In addition, anandamide activates vanilloid VR1 receptors, (a known alternative target of anandamide) present on sensory nerves triggering the release of calcitonin gene-related peptide that binds to its receptors to cause vasodilatation (13). The CB-2 receptors are expressed in cardiomyocytes, coronary endothelial cells and smooth muscle cells. Steffens and Pacher (14) examined the current literature on cannabinoid receptor CB-2 in cardiovascular disorders and concluded that expression of CB-2 receptors in cellular components of the cardiovascular system as well as infiltrating immune cells such as leukocytes and macrophages was possibly involved in controlling the extent of tissue inflammation and injury occurring in various cardiovascular conditions, thereby suggesting that these receptors may play a cardioprotective role. The pharmacological modulation of CB-2 receptors by CB-2 receptor agonists and antagonists therefore appears to be a promising strategy in the treatment of diseases such as stroke, atherosclerosis, restenosis, MI and heart failure. A thorough understanding of the endocannabinoid receptor system in humans would be paramount to the discovery of molecules that exert the therapeutic effects of cannabis and cannabinoids, with minimal adverse effects.
Arrhythmogenic properties of cannabis
One of the most consistent effects of cannabis smoking on heart is 20% to 100% increase in HR which can last up to 2–3 hours, often accompanied by a slight increase in supine blood pressure. This effect of cannabis on HR is thought to be due to cannabis induced vasodilation causing reflex tachycardia (15,16). Consumption of higher doses of cannabis can cause postural hypotension associated with dizziness or fainting (17,18). However, tolerance to the effects of cannabis develops rapidly after only a day or two of repeated exposure. Chronic marijuana use is associated with a decrease in HR, disappearance of orthostatic hypotension, increase in blood volume, and decrease in the circulatory responses to exercise which are consistent with reduced sympathetic and increased parasympathetic activity (18).
A study from Norway, on apprehended drivers, demonstrated that THC- positive drivers had higher mean pulse rate than THC-negative drivers and surprisingly, the magnitude of tachycardia was independent of blood THC concentration (19). Cannabis use has also been shown to be associated with development of atrial fibrillation especially in young patients who don’t have any risk factor (16,20). In a systematic review of 6 case reports, Korantzopoulos et al. (16) concluded that marijuana smoking might be associated with atrial fibrillation. They hypothesize that adrenergic stimulation reduces duration of action potential and alters the electrophysiological properties of myocardium to favour automaticity and micro-reentry thereby promoting development of atrial fibrillation in susceptible individuals. Moreover, atrial ischemia caused by detrimental effect of cannabis on coronary microcirculation could also contribute to development of atrial fibrillation (16). Ventricular tachycardia has been reported in a 29-year-old heart transplant patient, within the time frame of marijuana use documented by a 24-hour Holter Monitor (21). Casier et al. (22) also reported a case of fatal ventricular fibrillation in a chronic cannabis user following recent use of it. In another case, ventricular fibrillation occurred after consuming more than the usual dose of marijuana in a patient with CAD on two separate occasions. The authors speculated that excessive catecholamine release could be responsible for the arrhythmia (23). Brugada-like ST segment abnormalities have also been reported after heavy consumption of cannabis (24-26). This adverse effect of cannabis is thought to be due to its effect on shortening of action potential and hyper stimulation of vagal tone (24).
Thus, it appears that recent use of cannabis is associated with an increase in HR occurring in young individuals and potentially increasing the risk of sudden death. Since marijuana use is common among young persons in various social settings and the possibility of the combined use of marijuana with alcohol or other illicit substances is high, it is important to consider the potential additive or synergistic effects of commonly used such combinations. In a randomized, double blind, placebo controlled trial in healthy volunteers, Ballard et al. (27) found that combining very-low-dose ethanol and oral THC capsules (2.5 mg) did not show any synergistic effect on physiological responses or cognitive function in healthy volunteers. Assessed measures included psychomotor ability, simple reaction time and blood pressure and HR. However, in another randomized, double blind, placebo controlled trial, the combined use of “ecstasy” or 3,4-methylenedioxymethamphetamine (MDMA) and marijuana was examined by Dumont et al. (28) who showed that that both drugs had additive effects on increasing HR in healthy volunteers. THC co-administration also modulated the MDMA induced temperature increase by delaying and prolonging it.
Cannabis and acute coronary syndrome
In the past, risk of ischemia associated with marijuana use was considered to be low (18). Over the last few years, several case reports and case series have been published worldwide about the occurrence of MI in otherwise healthy young marijuana abusers most of which are particularly male. Hodcroft et al. (29) reported a case of marijuana-related MI triggered after sports activity in a patient who was a smoker but otherwise healthy. In a case series, Casier et al. (22) reported that marijuana smoking was associated with severe, potentially fatal, acute coronary events with documentation of the occurrence of diffuse coronary spasm and acute anterior coronary infarction. Deharo et al. (30), Ghannem et al. (31), Yurtdas and Aydin (32), and Canga et al. (33) also reported cases of exercise-induced coronary syndromes in previously healthy young men with history of regular and excessive use of marijuana as well as smoking cigarettes. Regular cannabis use was found to be linked with acute coronary syndrome after excessive physical activity and has been reported to trigger MI in patients with known coronary artery disease (34). Leblanc et al. (35) reported a case of recurrent ischemic stroke resulting from a post-MI left ventricular thrombus in a young man who was a heavy marijuana and tobacco smoker. Together, these case studies indicate that cannabis use is a risk factor for acute coronary events in young persons, especially men, who do not have any other risk factors for cardiovascular diseases besides smoking. The events also appear to be triggered by highly strenuous physical activity. This is an important consideration in youth who use marijuana in social entertainment settings since physical activity such as dancing may be common in such circumstances, which can increase the risk of precipitating acute coronary events.
From the Determinants of Myocardial Infarction Onset Study cohort, in a case-crossover study, Mittleman et al. (36) interviewed 3882 patients with acute myocardial infarction (AMI) to compare the reported of use of marijuana in the hour preceding symptoms onset to its expected frequency using self-matched control data. They found that marijuana smoking was associated with increased the risk of MI 4.8 times than baseline within one hour of use but this elevated risk appears to decrease rapidly thereafter. Further, the annual risk of MI in daily users of cannabis was calculated to be 1.5% to 3% per year (36). In another study from the similar cohort, 1913 patient were prospectively followed for an average of 3.8 years between 1989 and 1996 with 52 patients reported to consume marijuana within a year. The authors determined that compared to non-use, cannabis use less than a week, was associated with hazard ratio of 2.5 (95% CI, 0.9–7.3) and weekly or more use was associated with hazard ratio of 4.2 (95% CI, 1.2–14.3). Any cannabis use was associated with hazard ratio of 1.9 (95% CI, 0.6–6.3) for cardiovascular mortality and 4.9 (95% CI, 1.6–14.7) for non-cardiovascular mortality after age and sex adjustments (37). However, in a follow study from the same cohort, Frost et al. (38) could not demonstrate statistically significant association between cannabis use and mortality after 18 years of follow up.
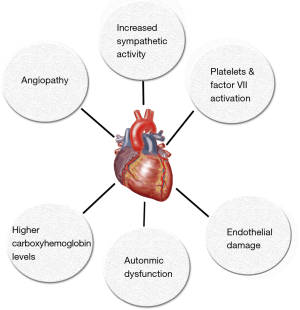
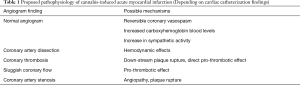
The mechanisms by which cannabis causes acute coronary syndrome are unknown and multiple hypothesis have been proposed (Table 1, Figure 1). In many reported cases of cannabis induced AMI coronary angiography was normal so cannabis-induced transient coronary vasospasm is one of the hypothesis proposed. In one case report, patient developed ST-segment elevation MI with clean coronaries after using combination of sildenafil and cannabis (39). Prolonged half-life of cannabis due to competitive substrate inhibition of CYP3A4 was proposed as the possible mechanism (39,40). Cannabis is also known to cause reversible cerebral vasoconstriction syndrome (RCVS) associated with or without focal neurological deficits where neurovascular imaging improves after discontinuation of cannabis use (discussed later in more detail). Another possible mechanism of AMI due to cannabis use could be increase in carboxyhemoglobin levels immediately after its inhalation which could reduce oxygen carrying capacity of blood. Aronow et al. (41) showed that smoking cannabis has acute effects on cardiovascular function and on exercise-induced angina in patients with angina pectoris. In their study, they found that smoking one marijuana cigarette increased the product of systolic blood pressure (SBP) and HR and decreased exercise angina threshold by 48% when compared to non-marijuana cigarettes which did not have an effect on double product and decreased exercise angina threshold by only 8.6%.
Full table
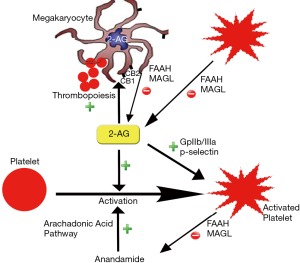
It has also been proposed that cannabis has pro-coagulant effects. Both CB1 and CB2 receptors have been detected on platelet cell membrane. It has been shown in vitro that cannabis increases expression of glycoprotein IIb-IIIa and P-selectin in a concentration dependent manner which leads to platelets aggregation and Factor VII activation (42) (Figure 2). Cannabis is also postulated to exert hemodynamic effects which could initiate plaque rupture and promote thrombosis. Increase in HR and sympathomimetic activity due to cannabis use can increase myocardial oxygen demand and could precipitate AMI. Postural hypotension and increase in blood pressure in supine position can also be precipitated by cannabis use which could trigger anginal episodes (22,36).
Thus, there is evidence to support the notion that cannabis use is associated with an increased risk of acute coronary events in patients with coronary artery disease as well as in those without any significant risk factors for atherosclerosis. However, some investigators believe that there is only low and transient risk of precipitating cardiovascular events associated with cannabis use considering that the reports of death among users of medical marijuana attributable to the drug are rare (43).
Peripheral vascular effects of cannabis
The effects of cannabis on peripheral vasculature have not been clinically well studied yet. Only a handful number of studies have been conducted on the relationship between blood vasomotion and cannabis and they show conflicting results. O’Sullivan et al. (44) showed that THC causes time dependent slow arterial relaxation in rats and that this effect was mediated by peroxisome proliferator-activated receptor-gamma (PPAR-γ). Further, their animal experiments revealed that in conduit arteries, such as the aorta, THC causes impairment of methoxamine-induced constriction under the influence of superoxide dismutase, production of hydrogen peroxide, and calcium channel inhibition. In the superior mesenteric artery, they noted that THC enhanced acetylcholine-induced vasorelaxation via increased hydrogen peroxide production. However, in isolated resistance vessels, THC augmented methoxamine-induced vasoconstriction and inhibited endothelium-dependent vasorelaxation (45). Subsequently, the same authors reported that cannabidiol also exerted its time-dependent vascular effects by binding to PPAR-γ (46). These findings suggest that THC has variable effects on the central and peripheral vessels, depending on the functional properties of the arteries examined. Vandrey et al. (47) conducted an intra-subject cross-over study and found that abrupt discontinuation of cannabis use resulted in an increase in mean SBP and diastolic blood pressure (DBP) of 22.8 and 12.3 mmHg respectively (47). In contrast, Bonnet et al. (48) recently showed in a post-hoc analysis that in patients treated for cannabis withdrawal syndrome, the abrupt cessation of marijuana was not associated with any significant alterations in blood pressure or HR. Alshaarawy and Elbaz (49) conducted a study using US National Health and Nutrition Examination Survey-NHANES (2005–2012) data, and found that active cannabis use was associated with increase in SBP in their sex-age adjusted model and no association between cannabis use and DBP was found. Cannabis smoking has also been shown to be associated with orthostatic hypotension which was more pronounced and longer lasting in hypertensive volunteers and is thought to be due to decreased vascular resistance (50).
An association between Cannabis use and arteritis has been suggested mainly as a cause of peripheral vascular disease in young patients <50 years of age (51). THC might possess direct toxic effect on arterial vessels and could exert a synergistic effect with tobacco smoking (52). THC induced peripheral vasoconstriction could also contribute to this phenomenon (51). Multiple case reports of cannabis associated arteritis have been published in literature, however, the casualty has not been well established because most of these cases had other risk factors for thromboangitis obliterans (34,53). In many instances, however, the worsening of the vascular disease was found to parallel the use of cannabis with halting of disease progression with abstinence from cannabis consumption (54). Lou et al. (55) reported a rare case of spontaneous dissection of the renal artery in a cannabis user. They proposed chronic vasoconstriction, as the possible underlying mechanism by causing partial or complete vacuolization and lysis of the arterial media with deposition of fibrin at the interface between the adventitia and the media, eventually resulting in arterial rupture. There are anecdotal case reports of central retinal vein occlusion (56), limb ischemia (57), and acute thrombosis of aorta (58) associated with cannabis use.
Thus, the effect of cannabinoids on peripheral vasculature is heterogeneous and complex. Further research in this field is required to understand the long-term effects of cannabis consumption on the peripheral vasculature and blood flow.
Effects on cerebrovascular system
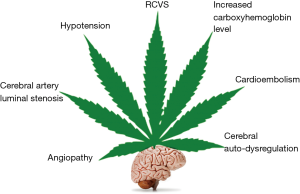
Currently, the evidence regarding the relationship between stroke and cannabis use is not firmly established, although a temporal link has been reported in several cases of ischemic stroke with no other apparent causes (59). Rumalla et al. (60) conducted a study in patients aged 18–54 years and found that recreational use of marijuana was independently associated with a 17% increase in risk of hospitalization due to acute ischemic stroke. They also found that the incidence of acute ischemic stroke was greater among marijuana users than among non-users. Similarly, a prospective study by Wolff et al. (61) on the relationship between ischemic stroke and cannabis use revealed that cannabis use was associated with multifocal angiopathy resulting in ischemic stroke in young individuals. Cannabis-related stroke can also occur as a consequence of arterial obstruction from a post-MI left ventricular thrombus, as reported by Leblanc et al. (35). Similarly, Tsivgoulis et al. (62) reported a case of transient ischemic attacks manifested as acute, transient episodes of hemiparesis in a patient with a history of daily cannabis consumption. In a systematic review of case reports on stroke and cannabis use by Hackam (63) showed that current evidences point towards a temporal relationship between ischemic stroke and cannabis use. They also found that about 22% of these patients had another ischemic stroke after subsequent re-exposure to cannabis which again strengthens the link between stroke and cannabis use. Cannabis related ischemic stroke has been found to have predilection for posterior circulation (64) which could be due to multifocal intracranial stenosis in vertebrobasilar territory (61). Thanvi and Treadwell (59) listed the following as possible mechanisms underlying the occurrence of stroke after cannabis consumption: vasospasm, vasculitis, hypotension with secondary impairment of regulation of cerebral flow and cerebral vasoconstriction syndrome (Figure 3). Ntlholang et al. (65) reported a few cases of stroke in cannabis users wherein they were able to obtain histological evidence showing gross hyperplasia of the tunica media leading to luminal stenosis of cerebral arteries. Recently, it was shown in rats that THC exposure induces cerebral mitochondrial dysfunction in dose dependent manner and increases reactive oxygen species production in the brain which could further contributes to its toxicity (66). This apparent relationship of cannabis use and stroke notwithstanding, it should also be noted that stroke is not commonly reported in cannabis users (59).
While several reports have indicated the occurrence of ischemic stroke associated with cannabis use but occurrence of hemorrhagic stroke has been rarely reported. Ince et al. (67) reported a case of both hemorrhagic and ischemic strokes subsequent to marijuana consumption in high doses. In a study from Nationwide Inpatient Sample (NIS) database (2004–2011), cannabis use was found to be independently associated with 18% increased likelihood of development of aneurysmal subarachnoid hemorrhage (aSAH) (68). Behrouz et al. (69) performed a retrospective study on 108 patients with aSAH out of which 25.9% were found to be positive for cannabis on urine drug screen. They found that about half of the cannabis positive patients developed delayed cerebral ischemia vs. only 23.8% in cannabis negative group (P=0.01). However, in another observational study in patients with spontaneous intracerebral hemorrhage (ICH), prior cannabis use was associated with lower admission ICH score (P=0.017) and less disability at discharge in terms of modified Rankin scale (70).
Cannabis use can also cause RCVS which is characterized by recurrent strong headaches and development of neurological focal deficit with reversible vasoconstriction on repeat intravascular imaging within three months (71). In a prospective series of 67 patients with RCVS, 20 patients (32%) were found to cannabis users with cannabis being the most common vasoactive drug used in the cohort (72). Wolf et al. showed that ischemic stroke due to reversible vasoconstriction without thunderclap headache was precipitated by cannabis use in some individuals (73,74).
It is possible that cannabis use is underdiagnosed or underreported in young adults developing ischemic and occasionally hemorrhagic stroke. Therefore, the actual magnitude of the contribution of cannabis usage to the incidence of stroke among youth may be significantly underestimated. This highlights the need for proper and thorough history taking in such cases focusing on the history use of recent or chronic use of marijuana, especially in the absence of other risk factors for stroke. A high degree of suspicion of illicit drug use, especially of cannabis, should be maintained in young patients presenting with stroke especially when no apparent cause of stroke can be found.
Other reported adverse cardiac effects of cannabis use
Takotsubo cardiomyopathy
Rarely, development of stress cardiomyopathy has been temporally related to consumption of cannabis (75). Recurrent stress cardiomyopathy involving cardiac apical and basal cardiac regions on two separate occasions in the same patient has also been reported. Because of involvement of two separate cardiac regions, authors questioned the hypothesis of reginal variability of β-adrenergic receptor density and sensitivity, and proposed that this phenomenon could be mediated by ECS (76).
Myopericarditis
A case of recurrent myopericarditis was reported in a 29-year-old male which occurred after heavy consumption of adulterated cannabis both times. Authors could not find any obvious causative factor other than use of cannabis (77).
Synthetic marijuana and its cardiovascular complications
Synthetic cannabinoids (SCs) are cannabis preparations that were synthesized during the process of identifying cannabinoid receptors. They were first sold under the name “spice” which is made of cannabinomimetic JWH-018 and other cannabinoids such as CP-47, 497,497-C8 (78). JWH-018 has stronger affinity for CB1 and CB2 receptors and produces extreme cannabinomimetic effects compared to marijuana. SCs are marketed under various other names including K2, skunk, joker, mojo, aroma, dream and black mamba etc. (78,79) and have gained increasing popularity because they are considered “legal” alternatives to marijuana that is safe (80). The rising popularity of these products has been accompanied by a significant increase in the number of emergency admissions due to SCs. In fact, in a report from Drug Abuse Warning Network (DAWN) in US, the number of emergency department visits specifically linked to SCs increased more than 2.5 times from 11,406 visits in 2010 to 28,531 visits in 2011 (81). There is considerable risk of toxicity from either acute or chronic use with evidence of an increase in the number of chronic or even daily users of this class of drugs and a parallel increase in cases of withdrawal complications (82).
Although widely considered harmless, several recent reports have been published on cases of serious cardiovascular events attributed to the use of SCs. The most prevalent cardiac side effect of SC consumption is tachycardia (83). The tachycardia is associated with elevated blood pressure in 1/3 to 3/4 of the cases (83,84). Chest pain has also been reported to occur after consumption of SCs (83). Other reported cardiovascular events are peri-mesencephalic sub-arachnoid hemorrhage and middle cerebral artery occlusion (83). Cases of ST-elevation MI, although rare, have been reported following the use of K2 in patients as young as 14 years old as well as in adults (85). On the other hand, Orsini et al. reported a case of non-ST-segment elevation MI leading to acute congestive heart failure (CHF) and acute hypoxemic respiratory failure, after consumption of SCs (78). They hypothesized that consumption of K2 caused transient myocardial ischemia resulting in ventricular stunning leading to acute CHF (78). Shah et al. (79) reported a case of AMI with left ventricular apical thrombus that could only be attributed to smoking marijuana and Spice in a 24-year-old male patient. Development of acute ischemic strokes due to consumption of SCs has also been reported in young adults without any history of predisposing factors (85).
Thus, there is growing concern regarding the increasing popularity of SCs because of their being marketed as “legal marijuana” among the youth, especially teenagers. This highlights importance of appropriate and complete history taking when patients in this age group present to the emergency department especially with cardiovascular events. In an effort to curb the sales and consumption of SCs, Drug Enforcement Administration (DEA) classified several of these substances as Class I schedule drugs. Important legal considerations relevant to the use of SCs are the lack of sufficiently reliable tests for their detection in urine, unlike those available for marijuana and the classification of these drugs as analogues of controlled substances rather than controlled substances (86). Efforts to curb its use via the implementation of appropriate public health strategies are imperative.
Pre- and perioperative implications of cannabis use
Cannabis users may require surgery due to injuries or accidents occurring after recent use. Cannabis has been shown to cause significant respiratory symptoms and changes in spirometry even with relatively short duration of inhalation (87). Cannabis use has been associated with significant airway inflammation and alteration in histopathology in bronchial mucosa and these effects appears to be additive when cannabis is smoked in conjunction with tobacco (88). In one study, it was concluded that smoking of cannabis is associated with significant airway inflammation which was similar to what encountered in tobacco smokers (89). Cannabis inhalation causes increased prevalence of chronic cough/chronic bronchitis, wheezing and shortness of breath with increased clinic visits for acute respiratory illnesses (90). Because of all these physiological and histopathological changes, patients undergoing surgery should be inquired about illicit drug use including cannabis (91,92).
The interactions between cannabis and anaesthetic agents and the effects of these interactions are poorly understood. In a prospective, randomized, single blinded study, regular cannabis users showed variable response to induction of anaesthesia with propofol when compared to non-users, although higher doses of propofol were needed to achieve loss of consciousness, adequate jaw relaxation and depression of airway reflexes for insertion of laryngeal mask (93). THC has also been reported to prolong the sedative effects of general anaesthesia in experimental models (94,95), and has been implicated in perioperative complications such as bronchospasm due to airway irritation, tachycardia, and uvular oedema (96). Cannabis leaves burn at higher temperature than similar quantity of tobacco causing increased direct airway irritation. Excessive respiratory burden of carbon monoxide and tar can occur with cannabis smoking when compared to smoking (91). Cannabis use is also possibly reported to be associated with diffuse alveolar haemorrhage in post-operative period in a patient which was thought to be due to (97) negative pressure pulmonary edema and possible inhibition of thrombin-driven clot formation (98).
It is important to extract history of cannabis use as a routine part of preoperative work up. The choice of the appropriate anaesthetic agent is important in cases of cannabis users. If sedative hypnotic drugs are used in cannabis users, excessive depression of the central nervous system may occur; therefore, barbiturates, opioids, and benzodiazepines, and phenothiazines are preferably avoided. Further, recent use of cannabis can cause decrease blood pressure due to vasodilatation along with tachycardia leading to increased oxygen myocardial demand (99), therefore drugs which are likely to increase HR, such as ketamine, atropine, and epinephrine should also be avoided (100). It should also keep in mind that the intraoperative and immediate postoperative need of opiates for analgesia in patients with history of recent or chronic cannabis consumption may be significantly increased (92,101).
Thus, complete illicit drug use history and tests to confirm cannabis use if suspected, are necessary before surgical interventions It may be difficult to obtain truthful and adequate data specially because of patients’ reticent but a higher degree of suspicion of cannabis use is justified in patients with chronic pain, h/o illicit drug use, or alcoholism.
Potential beneficial effects of cannabis in cardiovascular system
While the majority of published data suggest a harmful effect of cannabis and cannabinoids on the cardiovascular system, a few suggest possible beneficial effects. The use of cannabis or marijuana has been linked to increase risk of cardiac events immediately after use, although little information is available about the long-term impact of marijuana among patients with established coronary disease. An analysis carried out on around 4,000 MI patients from a U.S. multicenter, cohort study that were followed for up to 18 years, failed to show statistically significant differences in all-cause mortality (38).
Indirect beneficial effects have been demonstrated in studies showing that cannabis or marijuana use attenuates or modulates common cardiovascular disease risk factors. Preliminary data from a small double blinded placebo controlled study carried out in the U.K. examining the effects of cannabidiol delivered through an inhaler on cigarette consumption among smokers wishing to quit smoking, suggests a 40% smoking reduction in the treatment group compared to placebo (102). Additionally, a number of epidemiologic studies have shown lower prevalence of obesity and diabetes mellitus among marijuana users compared with those who never used marijuana, suggesting a relationship between cannabinoids and metabolic processes. A study done on 4657 adult Americans from the National Health and Nutrition Examination Survey showed that marijuana use was associated with lower levels of fasting insulin and HOMA-IR, and smaller waist circumference (103). Furthermore, some studies hypothesized that lower rates of obesity among habitual marijuana users are directly related to the exposure to the THC present in cannabis, and proposed its potential use for the management of obesity and its complications (104).
A recently published study on mice that tested three regimens of THC administration suggests that a pre-treatment with an ultra-low dose of THC provides a significant protection against an ischemic insult to the heart as evidenced by lower troponin levels, and reduced infarct size (105).
Conclusions
The fact that cannabis use has become increasingly popular among youngsters is a major cause for concern. It is important to consider a negative impact of cannabis abuse on education as well as the risk of abuse of other illicit drugs among the youth on the development of psychosis. Currently, there is a lack of consensus on what position to adopt regarding legalization of cannabis. While one view regards recreational cannabis uses as harmless, the opposing viewpoint is that it raises some serious public health concerns and that its use should continue to be discouraged by governing bodies and prohibited by law (Table 2). The literature suggests the occurrence of harmful effects including fatal cardiovascular events that could be related to cannabis use. Further research and studies are needed to determine the impact of acute and especially the chronic regular use of cannabis on various organ systems, particularly the cardiovascular system.

Full table
With the recent decriminalization and legalisation of cannabis use in some parts of the world and the increase in the number of conditions that cannabis can be prescribed for, there is a good possibility that physicians will encounter more cases of cardiovascular and cerebrovascular complications of cannabis use in the near future. It is necessary to increase awareness among physicians and the general public alike regarding the increased risk of cardiovascular complications associated with cannabis use. In addition, implementing effective strategies for the prudent dispersal of the drug is necessary to avoid unnecessary increases in cannabis-related complications and therefore preclude the resultant burden on public and private health services. The current evidences highlight the urgent need for a change in the mindset among cannabis users, particularly the young regarding the adverse effects of cannabis use and the risk of acute coronary events, stroke, and possibly death.
Acknowledgements
Authors would like to acknowledge Dr. Amar Shere MD for creating the online versions of all figures.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Crime UNOoDa. World Drug Report 2016. Available online: http://www.unodc.org/wdr2016/
- Green KM, Johnson RM, Milam AJ, et al. Racial differences and the role of neighborhood in the sequencing of marijuana and tobacco initiation among urban youth. Subst Abus 2016;37:507-10. [Crossref] [PubMed]
- National Conference of State Legislatures. . Accessed October 10, 2016.www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
- Hughes A, Lipari RN, Williams M. State Estimates of Adolescent Marijuana Use and Perceptions of Risk of Harm From Marijuana Use: 2013 and 2014. The CBHSQ Report Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2013-2015 Dec 17 2015.
- Hoch E, Bonnetn U, Thomasius R, et al. Risks associated with the non-medicinal use of cannabis. Dtsch Arztebl Int 2015;112:271-8. [PubMed]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet 2009;374:1383-91. [Crossref] [PubMed]
- Milroy CM, Parai JL. The histopathology of drugs of abuse. Histopathology 2011;59:579-93. [Crossref] [PubMed]
- Jouanjus E, Lapeyre-Mestre M, Micallef J, et al. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc 2014;3:e000638. [Crossref] [PubMed]
- Gaoni Y, Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J Am Chem Soc 1964;86:1646-47. [Crossref]
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 2002;54:161-202. [Crossref] [PubMed]
- Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 2015;36:277-96. [Crossref] [PubMed]
- Montecucco F, Di Marzo V. At the heart of the matter: the endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol Sci 2012;33:331-40. [Crossref] [PubMed]
- Hiley CR, Ford WR. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling. Biol Rev Camb Philos Soc 2004;79:187-205. [Crossref] [PubMed]
- Steffens S, Pacher P. Targeting cannabinoid receptor CB(2) in cardiovascular disorders: promises and controversies. Br J Pharmacol 2012;167:313-23. [Crossref] [PubMed]
- Franz CA, Frishman WH. Marijuana Use and Cardiovascular Disease. Cardiol Rev 2016;24:158-62. [Crossref] [PubMed]
- Korantzopoulos P, Liu T, Papaioannides D, et al. Atrial fibrillation and marijuana smoking. Int J Clin Pract 2008;62:308-13. [Crossref] [PubMed]
- Sidney S. Cardiovascular consequences of marijuana use. J Clin Pharmacol 2002;42:64S-70S. [Crossref] [PubMed]
- Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol 2002;42:58S-63S. [Crossref] [PubMed]
- Khiabani HZ, Mørland J, Bramness JG. Frequency and irregularity of heart rate in drivers suspected of driving under the influence of cannabis. Eur J Intern Med 2008;19:608-12. [Crossref] [PubMed]
- Korantzopoulos P. Marijuana smoking is associated with atrial fibrillation. Am J Cardiol 2014;113:1085-6. [Crossref] [PubMed]
- Sánchez Lázaro IJ, Almenar Bonet L, Sancho-Tello MJ, et al. Ventricular tachycardia due to marijuana use in a heart transplant patient. Rev Esp Cardiol 2009;62:459-61. [PubMed]
- Casier I, Vanduynhoven P, Haine S, et al. Is recent cannabis use associated with acute coronary syndromes? An illustrative case series. Acta Cardiol 2014;69:131-6. [Crossref] [PubMed]
- Baranchuk A, Johri AM, Simpson CS, et al. Ventricular fibrillation triggered by marijuana use in a patient with ischemic cardiomyopathy: a case report. Cases J 2008;1:373. [Crossref] [PubMed]
- Daccarett M, Freih M, Machado C. Acute cannabis intoxication mimicking brugada-like ST segment abnormalities. Int J Cardiol 2007;119:235-6. [Crossref] [PubMed]
- Pratap B, Korniyenko A. Toxic effects of marijuana on the cardiovascular system. Cardiovasc Toxicol 2012;12:143-8. [Crossref] [PubMed]
- Alonso JV, Teo BH, Pozo FJ, et al. Brugada electrocardiogram pattern induced by cannabis; is cannabis safe? Am J Emerg Med 2016;34:1738.e1-4.
- Ballard ME, de Wit H. Combined effects of acute, very-low-dose ethanol and delta(9)-tetrahydrocannabinol in healthy human volunteers. Pharmacol Biochem Behav 2011;97:627-31. [Crossref] [PubMed]
- Dumont GJ, Kramers C, Sweep FC, et al. Cannabis coadministration potentiates the effects of "ecstasy" on heart rate and temperature in humans. Clin Pharmacol Ther 2009;86:160-6. [Crossref] [PubMed]
- Hodcroft CJ, Rossiter MC, Buch AN. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J Emerg Med 2014;47:277-81. [Crossref] [PubMed]
- Deharo P, Massoure PL, Fourcade L. Exercise-induced acute coronary syndrome in a 24-year-old man with massive cannabis consumption. Acta Cardiol 2013;68:425-8. [Crossref] [PubMed]
- Ghannem M, Belhadj I, Tritar A, et al. Ann Cardiol Angeiol (Paris) 2013;62:424-8. [Cannabis and acute coronary syndrome with ST segment elevation]. [Crossref] [PubMed]
- Yurtdaş M, Aydın MK. Acute myocardial infarction in a young man; fatal blow of the marijuana: a case report. Korean Circ J 2012;42:641-5. [Crossref] [PubMed]
- Canga Y, Osmonov D, Karataş MB, et al. Cannabis: a rare trigger of premature myocardial infarction. Anadolu Kardiyol Derg 2011;11:272-4. [PubMed]
- Panayiotides IM. What is the Association of Cannabis Consumption and Cardiovascular Complications? Subst Abuse 2015;9:1-3. [Crossref] [PubMed]
- Leblanc A, Tirel-Badets A, Paleiron N, et al. Ann Cardiol Angeiol (Paris) 2011;60:154-8. [Cannabis and myocardial infarction without angiographic stenosis in young patient: guilty or not guilty? A case report]. [Crossref] [PubMed]
- Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation 2001;103:2805-9. [Crossref] [PubMed]
- Mukamal KJ, Maclure M, Muller JE, et al. An exploratory prospective study of marijuana use and mortality following acute myocardial infarction. Am Heart J 2008;155:465-70. [Crossref] [PubMed]
- Frost L, Mostofsky E, Rosenbloom JI, et al. Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am Heart J 2013;165:170-5. [Crossref] [PubMed]
- Arora S, Goyal H, Aggarwal P, et al. ST-segment elevation myocardial infarction in a 37-year-old man with normal coronaries--it is not always cocaine! Am J Emerg Med 2012;30:2091.e3-5. [Crossref]
- McLeod AL, McKenna CJ, Northridge DB. Myocardial infarction following the combined recreational use of Viagra and cannabis. Clin Cardiol 2002;25:133-4. [Crossref] [PubMed]
- Aronow WS, Cassidy J. Effect of marihuana and placebo-marihuana smoking on angina pectoris. N Engl J Med 1974;291:65-7. [Crossref] [PubMed]
- Deusch E, Kress HG, Kraft B, et al. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth Analg 2004;99:1127-30. table of contents. [Crossref] [PubMed]
- Karch SB. Cannabis and cardiotoxicity. Forensic Sci Med Pathol 2006;2:13-8. [Crossref] [PubMed]
- O'Sullivan SE, Tarling EJ, Bennett AJ, et al. Novel time-dependent vascular actions of Delta9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochem Biophys Res Commun 2005;337:824-31. [Crossref] [PubMed]
- O'Sullivan SE, Kendall DA, Randall MD. Further characterization of the time-dependent vascular effects of delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 2006;317:428-38. [Crossref] [PubMed]
- O'Sullivan SE, Sun Y, Bennett AJ, et al. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur J Pharmacol 2009;612:61-8. [Crossref] [PubMed]
- Vandrey R, Umbricht A, Strain EC. Increased blood pressure after abrupt cessation of daily cannabis use. J Addict Med 2011;5:16-20. [Crossref] [PubMed]
- Bonnet U. Abrupt Quitting of Long-term Heavy Recreational Cannabis Use is Not Followed by Significant Changes in Blood Pressure and Heart Rate. Pharmacopsychiatry 2016;49:23-5. [Crossref] [PubMed]
- Alshaarawy O, Elbaz HA. Cannabis use and blood pressure levels: United States National Health and Nutrition Examination Survey, 2005-2012. J Hypertens 2016;34:1507-12. [Crossref] [PubMed]
- Malinowska B, Baranowska-Kuczko M, Schlicker E. Triphasic blood pressure responses to cannabinoids: do we understand the mechanism? Br J Pharmacol 2012;165:2073-88. [Crossref] [PubMed]
- Grotenhermen F. Cannabis-associated arteritis. Vasa 2010;39:43-53. [Crossref] [PubMed]
- Karila L, Roux P, Rolland B, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014;20:4112-8. [Crossref] [PubMed]
- Cottencin O, Karila L, Lambert M, et al. Cannabis arteritis: review of the literature. J Addict Med 2010;4:191-6. [Crossref] [PubMed]
- Peyrot I, Garsaud AM, Saint-Cyr I, et al. Cannabis arteritis: a new case report and a review of literature. J Eur Acad Dermatol Venereol 2007;21:388-91. [Crossref] [PubMed]
- Lou JY, Randhawa MS, Hornacek D, et al. Images in vascular medicine. Spontaneous renal artery dissection in a cannabis user. Vasc Med 2015;20:379-80. [Crossref] [PubMed]
- Corvi F, Querques G, Lattanzio R, et al. Central retinal vein occlusion in a young patient following cannabis smoke inhalation. Eur J Ophthalmol 2014;24:437-40. [Crossref] [PubMed]
- Bucci F, Redler A, Fiengo L. Critical limb ischemia in a young man: saddle embolism or unusual presentation of thromboangiitis obliterans? Case Rep Vasc Med 2013;2013:830540. [Crossref] [PubMed]
- Ulenaers M, Buchel OC, Van Olmen A, et al. An uncommon cause of visceral arterial embolism in patients presenting with acute abdominal pain: a report of 2 cases. Acta Gastroenterol Belg 2010;73:55-60. [PubMed]
- Thanvi BR, Treadwell SD. Cannabis and stroke: is there a link? Postgrad Med J 2009;85:80-3. [Crossref] [PubMed]
- Rumalla K, Reddy AY, Mittal MK. Recreational marijuana use and acute ischemic stroke: A population-based analysis of hospitalized patients in the United States. J Neurol Sci 2016;364:191-6. [Crossref] [PubMed]
- Wolff V, Lauer V, Rouyer O, et al. Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke 2011;42:1778-80. [Crossref] [PubMed]
- Tsivgoulis G, Lachanis S, Papathanasiou MA, et al. Cannabis-associated angiopathy: an uncommon cause of crescendo transient ischemic attacks. Circulation 2014;130:2069-70. [Crossref] [PubMed]
- Hackam DG. Cannabis and stroke: systematic appraisal of case reports. Stroke 2015;46:852-6. [Crossref] [PubMed]
- Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg 2013;27:996-1005. [Crossref] [PubMed]
- Ntlholang O, McDonagh R, Nicholson S, et al. Is intimal hyperplasia associated with cranial arterial stenosis in cannabis-associated cerebral infarction? Int J Stroke 2015;10:E56-9. [Crossref] [PubMed]
- Wolff V, Schlagowski AI, Rouyer O, et al. Tetrahydrocannabinol induces brain mitochondrial respiratory chain dysfunction and increases oxidative stress: a potential mechanism involved in cannabis-related stroke. Biomed Res Int 2015;2015:323706. [Crossref] [PubMed]
- Ince B, Benbir G, Yuksel O, et al. Both hemorrhagic and ischemic stroke following high doses of cannabis consumption. Presse Med 2015;44:106-7. [Crossref] [PubMed]
- Rumalla K, Reddy AY, Mittal MK. Association of Recreational Marijuana Use with Aneurysmal Subarachnoid Hemorrhage. J Stroke Cerebrovasc Dis 2016;25:452-60. [Crossref] [PubMed]
- Behrouz R, Birnbaum L, Grandhi R, et al. Cannabis Use and Outcomes in Patients With Aneurysmal Subarachnoid Hemorrhage. Stroke 2016;47:1371-3. [Crossref] [PubMed]
- Di Napoli M, Zha AM, Godoy DA, et al. Prior Cannabis Use Is Associated with Outcome after Intracerebral Hemorrhage. Cerebrovasc Dis 2016;41:248-55. [Crossref] [PubMed]
- Wolff V, Armspach JP, Lauer V, et al. Cannabis-related stroke: myth or reality? Stroke 2013;44:558-63. [Crossref] [PubMed]
- Ducros A, Boukobza M, Porcher R, et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain 2007;130:3091-101. [Crossref] [PubMed]
- Wolff V, Armspach JP, Lauer V, et al. Ischaemic strokes with reversible vasoconstriction and without thunderclap headache: a variant of the reversible cerebral vasoconstriction syndrome? Cerebrovasc Dis 2015;39:31-8. [Crossref] [PubMed]
- Wolff V, Ducros A. Reversible Cerebral Vasoconstriction Syndrome Without Typical Thunderclap Headache. Headache 2016;56:674-87. [Crossref] [PubMed]
- Ting JY. Reversible cardiomyopathy associated with acute inhaled marijuana use in a young adult. Clin Toxicol (Phila) 2007;45:432-4. [Crossref] [PubMed]
- Kaushik M, Alla VM, Madan R, et al. Recurrent stress cardiomyopathy with variable regional involvement: insights into etiopathogenetic mechanisms. Circulation 2011;124:e556-7. [Crossref] [PubMed]
- Rodríguez-Castro CE, Alkhateeb H, Elfar A, et al. Recurrent myopericarditis as a complication of Marijuana use. Am J Case Rep 2014;15:60-2. [Crossref] [PubMed]
- Orsini J, Blaak C, Tam E, et al. The Wide and Unpredictable Scope of Synthetic Cannabinoids Toxicity. Case Rep Crit Care 2015;2015:542490. [Crossref] [PubMed]
- Shah M, Garg J, Patel B, et al. Can your heart handle the spice: A case of acute myocardial infarction and left ventricular apical thrombus. Int J Cardiol 2016;215:129-31. [Crossref] [PubMed]
- Castaneto MS, Gorelick DA, Desrosiers NA, et al. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend 2014;144:12-41. [Crossref] [PubMed]
- Bush DM, Woodwell DA. Update: Drug-Related Emergency Department Visits Involving Synthetic Cannabinoids. The CBHSQ Report Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2013-2014 Oct 16 2013-2014.
- Cooper ZD. Adverse Effects of Synthetic Cannabinoids: Management of Acute Toxicity and Withdrawal. Curr Psychiatry Rep 2016;18:52. [Crossref] [PubMed]
- Tait RJ, Caldicott D, Mountain D, et al. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila) 2016;54:1-13. [Crossref] [PubMed]
- Obafemi AI, Kleinschmidt K, Goto C, et al. Cluster of Acute Toxicity from Ingestion of Synthetic Cannabinoid-Laced Brownies. J Med Toxicol 2015;11:426-9. [Crossref] [PubMed]
- Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus 2016.1-23. [Crossref] [PubMed]
- Wells DL, Ott CA. The "new" marijuana. Ann Pharmacother 2011;45:414-7. [Crossref] [PubMed]
- Taylor DR, Poulton R, Moffitt TE, et al. The respiratory effects of cannabis dependence in young adults. Addiction 2000;95:1669-77. [Crossref] [PubMed]
- Fligiel SE, Roth MD, Kleerup EC, et al. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest 1997;112:319-26. [Crossref] [PubMed]
- Roth MD, Arora A, Barsky SH, et al. Airway inflammation in young marijuana and tobacco smokers. Am J Respir Crit Care Med 1998;157:928-37. [Crossref] [PubMed]
- Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013;10:239-47. [Crossref] [PubMed]
- Wong GT, Irwin MG. Poisoning with illicit substances: toxicology for the anaesthetist. Anaesthesia 2013;68 Suppl 1:117-24. [Crossref] [PubMed]
- Karam K, Abbasi S, Khan FA. Anaesthetic consideration in a cannabis addict. J Coll Physicians Surg Pak 2015;25 Suppl 1:S2-3. [PubMed]
- Flisberg P, Paech MJ, Shah T, et al. Induction dose of propofol in patients using cannabis. Eur J Anaesthesiol 2009;26:192-5. [Crossref] [PubMed]
- Chesher GB, Jackson DM, Starmer GA. Interaction of cannabis and general anaesthetic agents in mice. Br J Pharmacol 1974;50:593-9. [Crossref] [PubMed]
- Frizza J, Chesher GB, Jackson DM, et al. The effect of delta 9-tetrahydrocannabinol, cannabidiol, and cannabinol on the anaesthesia induced by various anaesthetic agents in mice. Psychopharmacology (Berl) 1977;55:103-7. [Crossref] [PubMed]
- Mallat A, Roberson J, Brock-Utne JG. Preoperative marijuana inhalation--an airway concern. Can J Anaesth 1996;43:691-3. [Crossref] [PubMed]
- Kim CA, Liu R, Hsia DW. Diffuse alveolar hemorrhage induced by sevoflurane. Ann Am Thorac Soc 2014;11:853-5. [Crossref] [PubMed]
- Murray AW, Smith JD, Ibinson JW. Diffuse alveolar hemorrhage, anesthesia, and cannabis. Ann Am Thorac Soc 2014;11:1338-9. [Crossref] [PubMed]
- Bryson EO, Frost EA. The perioperative implications of tobacco, marijuana, and other inhaled toxins. Int Anesthesiol Clin 2011;49:103-18. [Crossref] [PubMed]
- Hernandez M, Birnbach DJ, Van Zundert AA. Anesthetic management of the illicit-substance-using patient. Curr Opin Anaesthesiol 2005;18:315-24. [Crossref] [PubMed]
- Jefferson DA, Harding HE, Cawich SO, et al. Postoperative analgesia in the Jamaican cannabis user. J Psychoactive Drugs 2013;45:227-32. [Crossref] [PubMed]
- Morgan CJ, Das RK, Joye A, et al. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav 2013;38:2433-6. [Crossref] [PubMed]
- Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med 2013;126:583-9. [Crossref] [PubMed]
- Le Foll B, Trigo JM, Sharkey KA, et al. Cannabis and Δ9-tetrahydrocannabinol (THC) for weight loss? Med Hypotheses 2013;80:564-7. [Crossref] [PubMed]
- Waldman M, Hochhauser E, Fishbein M, et al. An ultra-low dose of tetrahydrocannabinol provides cardioprotection. Biochem Pharmacol 2013;85:1626-33. [Crossref] [PubMed]