Clinical and genetic study of a large Chinese family presented with familial spontaneous pneumothorax
Introduction
Primary spontaneous pneumothorax (PSP; OMIM 173600) is a condition in which air enters the pleural space, without obvious underlying lung disease. Approximately 11.5% of patients presenting with spontaneous pneumothorax report a positive family history of the condition (1). Familial spontaneous pneumothorax (FSP) was initially reported in 1921 by Faber (2). Several monogenetic disorders are associated with FSP, including Marfan syndrome, homocystinuria, Ehlers-Danlos syndrome, α1-antitrypsin deficiency and Birt-Hogg-Dubé (BHD) syndrome (3). A significant fraction of the reported FSP cases are associated with BHD syndrome (4-7).
BHD syndrome (OMIM 135150) is a rare autosomal dominantly hereditary disease, which was firstly described in 1977 by Birt et al. (8). This disease is characterized by skin fibrofolliculomas, pulmonary cyst, spontaneous pneumothorax, and kidney neoplasms (9). The phenotype of BHD syndrome is highly heterogeneous and varies among patients (10). Pulmonary cyst is one of the most common manifestations. About 80% of patients have multiple pulmonary cysts, as visible on chest computed tomography (CT), and about 30% of patients develop spontaneous pneumothorax (11). In 2001, the gene responsible for BHD syndrome was mapped to the short arm of chromosome 17 (17p11.2) and was named folliculin gene (FLCN, OMIN 607273, GenBank accession number NM_144997). This gene encodes folliculin (FLCN) protein, which is expressed in a variety of tissues, including the skin and its appendages, the distal nephron of the kidney, stromal cells, and type I pneumocytes of the lung (12,13). Thus far, over 100 germline mutations have been identified in all 14 exons of the FLCN gene (14). The correlation between the mutation genotype and phenotype is unclear (10).
Here, we reported a large Chinese family in which six members from five generations developed spontaneous pneumothorax. A genetic study revealed that 17 of the 35 members in this family harbored the same FLCN germline mutation. The clinical characteristics of BHD syndrome-related FSP were discussed. To the best of our knowledge, this is the largest single-family report on BHD syndrome-related FSP from China.
Methods
A large family from eastern China was recruited for this study. The family history of the members was obtained through the proband and several family members. The detailed clinical information of the six patients who developed spontaneous pneumothorax, including their medical history, smoking status, body weight and height, chest CT imaging result, and the choice of treatment choice were retrospectively reviewed. During health checkup, chest CT images of two other normal members were obtained, and all CT images were evaluated by thoracic radiologists from our hospital.
In total, 55 individuals underwent genetic analysis, namely 5 patients with pneumothorax, 30 normal family members (members related through marriage were excluded) and 20 unrelated healthy controls. Peripheral blood samples were obtained and genomic DNA was extracted from blood leukocytes according to standard procedures. The coding regions of the FLCN gene consisting of exon 4 to 14 and flanking introns were amplified using polymerase chain reaction (PCR) with oligonucleotide primers and were sequenced using the Sanger method (details are available upon request). Any detected sequences variants were compared with the reference sequences using Sequencher software (Gene Codes, Ann Arbor, MI, USA).
Patient characteristics were analyzed by basic descriptive data analysis, using SPSS 20.0 (IBM Corporation, Chicago, USA). Data were expressed as median with interquartile range (IQR), and count data were expressed as percentage.
Results
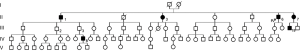
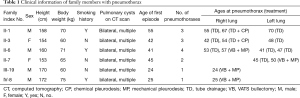
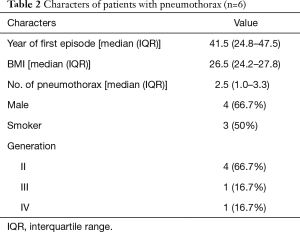
Figure 1 shows the simplified pedigree of this family (spouses are omitted). There were 5 generations and 76 members in this family. Six members, namely II-1, II-3, II-6, II-7, III-19, and IV-8 had experienced at least one episode of pneumothorax. The clinical information and characteristics of the six members with pneumothorax are listed in Tables 1 and 2. There was no known inherited disease or connective tissue disease among the members of the extended family. Moreover, none of the members showed any clinical signs or symptoms of Marfan syndrome or Ehlers-Danlos syndrome. Alpha-1 antitrypsin deficiency was also excluded. The median age for the initial onset of pneumothorax was 41.5 (IQR: 24.8–47.5) years, and the male to female ratio was 4:2. The median BMI was 26.5 (IQR: 24.2–27.8) kg/m2 and the median number of episodes was 2.5 (IQR: 1.0–3.3). All patients with pneumothorax showed bilateral multiple pulmonary cysts on CT imaging. Although III-2 and III-3 had never developed pneumothorax, chest CT showed multiple cysts in the bilateral lungs. The proband had no renal tumor or skin lesion, as confirmed by abdominal MRI and a thorough examination by a dermatologist. None of the 76 members reported a history of renal cancer. The dead members died of stroke (I-1 and II-2), heart failure (I-2), and breast cancer (III -9).
Full table
Full table
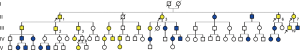
Sequence analysis of the FLCN gene revealed a deletion mutation (c.1285delC) in exon 11 (Figure 2). In total, 17 of the 35 family members harbored the same mutation (Figure 3). Of all the mutation carriers, nine were male and eight were female. Five of the 17 mutation carriers developed pneumothorax (29.4%). All members with spontaneous pneumothorax, except IV-8, whose blood sample was not obtained, showed positive results for the mutation test. Besides, III-2 and III-3, who showed pulmonary cysts on chest CT but did not develop pneumothorax, were also mutation carriers. None of the 20 unrelated healthy controls harbored this mutation.

Discussion
FSP accounts for approximately 11.5% of all PSP cases. Some small scale genetic studies on FSP have been reported. Jodie et al. reported a large single family with FSP from Finland and found a 4-bp deletion in the FLCN gene. In addition, all carriers of the mutation in this family had bullous lung lesions, as confirmed by HRCT (15). Ren et al. reported a cohort of patients with spontaneous pneumothorax from China and found that out of 10 unrelated patients with FSP, five patients were confirmed to harbor the FLCN mutation. They also demonstrated that FLCN mutation contributes to not only familial but also apparently sporadic patients with isolated spontaneous pneumothorax (7). Some other single-family reports of FSP from Korea (6), India (16) and the USA (17,18) also found different FLCN gene mutations.
The clustering of spontaneous pneumothorax in this family raised a concern regarding BHD syndrome. Using the sequence analysis, we identified a heterozygous C deletion in the poly-C tract in exon 11 cDNA nucleotide position 1285. This mutation was firstly reported in 2002 by Khoo et al. (19), and it is one of the most frequently reported mutations in BHD syndrome. This mutation is predicted to cause premature truncation of the FLCN protein 39 missense amino acids downstream. Loss of function of this protein can cause alveolar enlargement and cysts formation, consequently leading to pneumothorax. Recently, Elena et al. reported the possible molecular mechanism underlying lung epithelial cell apoptosis and alveolar enlargement, through E-cadherin, LKB1, and AMPK pathway (20).
In this family, we identified 17 members harboring this mutation. Pneumothorax was observed in 5 out of 17 (29.4%) members who carried a mutation in this family, which was similar to a prevalence of 29% in a previous report (21). However, according to Jodie et al., the bullous lung changes observed on chest CT scan should be considered as the pulmonary manifestation of this syndrome (15). The multiple pulmonary cysts observed in mutation carriers III-2 and III-3 support this idea. Therefore, it was difficult to calculate the accurate frequency of pulmonary involvement in this family without CT imaging; several previous reports with different sample sizes showed that the number ranges from 70% to 100% (15,22-24). Late onset may also play a role in the reported difference in pulmonary involvement. None of the healthy controls harbored this mutation, suggesting the rarity of this mutation in the normal population.
The genetic analysis confirmed the diagnosis of BHD syndrome in this family according to the diagnostic criteria (25). However, we did not find any renal tumor or skin lesion in the proband. The incomplete penetrance of BHD syndrome has been widely reported (10,22). Some genotypes are believed to be associated with certain clinical manifestations. For example, Toro et al. reported that FLCN mutation in exon 9 and 12 is associated with a higher number of pulmonary cysts, larger cyst diameter and more episodes of pneumothorax (11). Schmidt et al. reported that the frequency of renal neoplasm is significantly lower in patients with a deleted cytosine in exon 11 than in patients with an inserted cytosine in this exon (26). However, these genotype-phenotype correlations are generally not accepted. Further studies involving more patients are warranted to address this issue.
In the family of this study, 6 members developed 14 episodes of spontaneous pneumothorax altogether. The median age at initial onset was 41.5 years old and the median BMI was 26.5 kg/m2. Although more males developed pneumothorax (4:2), the distribution of mutation carriers in male and female patients was almost equal (9:8). These characteristics are different from those of primary spontaneous pneumothorax, which typically affects tall, thin males aged between 10 and 30 years (27). The treatment options for the 14 episodes of pneumothorax included chest tube drainage, chemical pleurodesis, VATS bullectomy and mechanical pleurodesis. The recurrence rate after chest tube drainage and chemical pleurodesis was 50% (5 in 10 episodes), while no recurrent pneumothorax occurred after VATS bullectomy and mechanical pleurodesis (0 in 4 episodes). Toro et al. reported that for BHD syndrome-related FSP, observation or tube drainage with or without chemical pleurodesis resulted in a significantly higher recurrence rate of 54.7% (35 in 64 episodes), compared with that observed after bullectomy combined with mechanical or chemical pleurodesis, which was 21.6% (8 in 37 episodes) (11). As for primary spontaneous pneumothorax, the average rate of recurrence after observation, needle aspiration or tube drainage is about 30% (28), which is lower than the rate of BHD syndrome-related FSP with these treatments. The difference in the recurrence rate may be explained by the multiple pulmonary cysts in patients with BHD syndrome-related pneumothorax.
The special characteristics of BHD syndrome-related FSP suggest that spontaneous pneumothorax is a heterogeneous disease group and needs to be stratified. Considering that over 10% of patients with spontaneous pneumothorax have a positive family history and the potential risk of developing renal cancer (1), we suggest that patients with spontaneous pneumothorax, especially those with a positive family history, should undergo the FLCN mutation screening, since genetic analysis is gradually becoming a routine test in more and more medical centers. Currently, there is no curative treatment for BHD syndrome. For patients who develop spontaneous pneumothorax, VATS bullectomy combined with pleurodesis seems to be a more effective choice of treatment. Taking into account of our experience and previously published data, early surgical intervention may be advised for BHD syndrome-related FSP. However, further prospective trials are warranted to guide the choice of treatment. Renal cancer is the most life-threatening complication in BHD syndrome, with a reported range of prevalence from 6.5% to 34% (9,26,29). Hence, we recommend annual screening for early detection of renal neoplasm in such patients.
This study has several limitations. First, we did not obtain the detailed clinical information of all family members, including skin examination, and chest and renal scan. This information could help us better understand the association between phenotype and genotype in BHD syndrome. Second, results from a single family and the retrospective nature of the study make it difficult to draw conclusion. Therefore, a prospective cohort with a larger sample size and detailed clinical information is required.
In conclusion, we reported a large family that presented with FSP. Genetic analysis found a germline mutation of FLCN gene in this family, which confirmed the diagnosis of BHD syndrome. To the best of our knowledge, this is the largest single-family report of BHD syndrome-related FSP from China, in which the clinical characteristics were discussed. Further studies are warranted to better understand the nature of this disease.
Acknowledgements
We thank all the members from this family for participating in this study. This study was supported by Peking University People’s Hospital Research and Development Funds.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Peking University People’s Hospital in China (No. 2014PHB061-01) and written informed consent was obtained from all patients.
References
- Abolnik IZ, Lossos IS, Zlotogora J, et al. On the inheritance of primary spontaneous pneumothorax. Am J Med Genet 1991;40:155-8. [Crossref] [PubMed]
- Faber. Spontaneous pneumothorax in 2 siblings. Hospital Stidende 1921;64:573-4.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006;12:268-72. [Crossref] [PubMed]
- Gunji Y, Akiyoshi T, Sato T, et al. Mutations of the Birt Hogg Dube gene in patients with multiple lung cysts and recurrent pneumothorax. J Med Genet 2007;44:588-93. [Crossref] [PubMed]
- Fröhlich BA, Zeitz C, Matyas G, et al. Novel mutations in the folliculin gene associated with spontaneous pneumothorax. Eur Respir J 2008;32:1316-20. [Crossref] [PubMed]
- Kim J, Yoo JH, Kang DY, et al. Novel in-frame deletion mutation in FLCN gene in a Korean family with recurrent primary spontaneous pneumothorax. Gene 2012;499:339-42. [Crossref] [PubMed]
- Ren HZ, Zhu CC, Yang C, et al. Mutation analysis of the FLCN gene in Chinese patients with sporadic and familial isolated primary spontaneous pneumothorax. Clin Genet 2008;74:178-83. [Crossref] [PubMed]
- Birt AR, Hogg GR, Dube WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977;113:1674-7. [Crossref] [PubMed]
- Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet 2008;45:321-31. [Crossref] [PubMed]
- Schmidt LS, Linehan WM. Molecular genetics and clinical features of Birt-Hogg-Dube syndrome. Nat Rev Urol 2015;12:558-69. [Crossref] [PubMed]
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dube syndrome. Am J Respir Crit Care Med 2007;175:1044-53. [Crossref] [PubMed]
- Schmidt LS, Warren MB, Nickerson ML, et al. Birt-Hogg-Dube syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. Am J Hum Genet 2001;69:876-82. [Crossref] [PubMed]
- Warren MB, Torres-Cabala CA, Turner ML, et al. Expression of Birt-Hogg-Dube gene mRNA in normal and neoplastic human tissues. Mod Pathol 2004;17:998-1011. [Crossref] [PubMed]
- Lim DH, Rehal PK, Nahorski MS, et al. A new locus-specific database (LSDB) for mutations in the folliculin (FLCN) gene. Hum Mutat 2010;31:E1043-51. [Crossref] [PubMed]
- Painter JN, Tapanainen H, Somer M, et al. A 4-bp deletion in the Birt-Hogg-Dube gene (FLCN) causes dominantly inherited spontaneous pneumothorax. Am J Hum Genet 2005;76:522-7. [Crossref] [PubMed]
- Ray A, Paul S, Chattopadhyay E, et al. Genetic analysis of familial spontaneous pneumothorax in an Indian family. Lung 2015;193:433-8. [Crossref] [PubMed]
- Graham RB, Nolasco M, Peterlin B, et al. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med 2005;172:39-44. [Crossref] [PubMed]
- Auerbach A, Roberts DH, Gangadharan SP, et al. Birt-Hogg-Dube syndrome in a patient presenting with familial spontaneous pneumothorax. Ann Thorac Surg 2014;98:325-7. [Crossref] [PubMed]
- Khoo SK, Giraud S, Kahnoski K, et al. Clinical and genetic studies of Birt-Hogg-Dube syndrome. J Med Genet 2002;39:906-12. [Crossref] [PubMed]
- Goncharova EA, Goncharov DA, James ML, et al. Folliculin controls lung alveolar enlargement and epithelial cell survival through E-cadherin, LKB1, and AMPK. Cell Rep 2014;7:412-23. [Crossref] [PubMed]
- Houweling AC, Gijezen LM, Jonker MA, et al. Renal cancer and pneumothorax risk in Birt-Hogg-Dube syndrome; an analysis of 115 FLCN mutation carriers from 35 BHD families. Br J Cancer 2011;105:1912-9. [Crossref] [PubMed]
- Kluger N, Giraud S, Coupier I, et al. Birt-Hogg-Dube syndrome: clinical and genetic studies of 10 French families. Br J Dermatol 2010;162:527-37. [Crossref] [PubMed]
- Skolnik K, Tsai WH, Dornan K, et al. Birt-Hogg-Dube syndrome: a large single family cohort. Respir Res 2016;17:22. [Crossref] [PubMed]
- Agarwal PP, Gross BH, Holloway BJ, et al. Thoracic CT findings in Birt-Hogg-Dube syndrome. AJR Am J Roentgenol 2011;196:349-52. [Crossref] [PubMed]
- Menko FH, van Steensel MA, Giraud S, et al. Birt-Hogg-Dube syndrome: diagnosis and management. Lancet Oncol 2009;10:1199-206. [Crossref] [PubMed]
- Schmidt LS, Nickerson ML, Warren MB, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dube syndrome. Am J Hum Genet 2005;76:1023-33. [Crossref] [PubMed]
- Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. [Crossref] [PubMed]
- Schramel FM, Postmus PE, Vanderschueren RG. Current aspects of spontaneous pneumothorax. Eur Respir J 1997;10:1372-9. [Crossref] [PubMed]
- Kunogi M, Kurihara M, Ikegami TS, et al. Clinical and genetic spectrum of Birt-Hogg-Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet 2010;47:281-7. [Crossref] [PubMed]


