The efficacy and toxicity of afatinib in advanced EGFR-positive non-small-cell lung cancer patients after failure of first-generation tyrosine kinase inhibitors: a systematic review and meta-analysis
Introduction
Lung cancer has become the second most common cancer and the leading cause of death in both men and women in US and the most common incidence and leading cause cancer in China (1,2). Non-small-cell lung cancer (NSCLC) counts for over 85% of all lung cancer (3). For all advanced NSCLC, the previous standard treatment is platinum-based doublet chemotherapy for four to six cycles (4). However, during the past few decades, epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have been proved to be an effective choice for patients with EGFR mutation. The clinical benefit of the targeting EGFR signaling pathways for advanced NSCLC patients has been validated proved (5,6). The first-generation TKIs have become the standard first-line treatment for NSCLC patients with EGFR mutation, including the reversible inhibitors, gefitinib and erlotinib. While the second-generation TKI, afatinib, was designed covalently bind to and irreversible inhibit active ErbB receptor family members, which can cause longer suppression in kinase activity than first generation reversible TKIs. The most common adverse effects of EGFR-TKIs included diarrhea, skin deformation, stomatitis (7), mucositis, and paronychia. Clinically grade 3/4 adverse effects may lead to a dose reduction or treatment stop, most commonly seen in diarrhea and skin toxicity (8,9).
The frequency of EGFR mutations depended on the histology and ethnicity, which can be as high as 30–40% in an East Asian population with adenocarcinoma (10). Most clinical relevant EGFR mutations occurred within the four exons encoding the ATP-binding pocket of the kinase domain (exons 18–21). The most common mutation sites were deletions in exon 19 (19 Del) and point mutation in exon 21 (21 L858R), accounting for about 85% of all EGFR mutations in lung cancer. The first generation TKIs drugs including gefitinib and erlotinib could reversibly bind to these mutations. Many trials had confirmed the significant initial treatment response and obviously delay in tumor progression (11,12). However, drug resistance became almost inevitable, nearly all patients developed progression after a 10 months of treatment (13,14). The most common cause of acquired resistance was the presence of the EGFR mutation T790M; accounting for about 50–60% of patients gained acquired resistance (9). Preclinical data suggested that afatinib is more active than first-generation EGFR TKIs in NSCLC cell lines harboring T790M mutations. Besides, in vitro experiments shows that afatinib was 100 folds more active than gefitinib in L858R/T790M double mutation patients in a cell free system (15). Since the tolerance was common in the first generation TKIs, the ability of afatinib irreversibly inhibiting EGFR and other targets within the ErbB family might improve upon first generation EGFR inhibitors and possibly overcome the resistance to these agents. Both in vitro and in vivo studies had suggested that afatinib was more active than first-generation EGFR-TKIs in cells or patients with T790M mutation. To the best of our knowledge, there was no pooled analysis to report the efficacy and toxicity of afatinib after the first generation TKIs in EGFR-positive patients. Therefore, a meta-analysis was conducted to solve the above question.
Methods
Search strategy and inclusion criteria
Literature search was conducted from the electronic databases in PubMed, Web of Science and Cochrane to before the end of June in 2016. The following search terms, treated as free text or mesh terms, were used: afatinib; NSCLC; EGFR; mutation; tyrosine kinase inhibitor; gefitinib; erlotinib. The search was restricted to human studies published in the English language. Abstracts from the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) conferences between January 2008 and June 2016 were also searched for relevant clinical trials. Studies that met the following criteria were included: (I) studies focused on advanced NSCLC; (II) patients with EGFR mutation or the data of EGFR mutation subgroup was shown; (III) failure of the first generation EGFR-TKIs (gefitinib or erlotinib) before admission of afatinib and (IV) at least one outcome available regarding the treatment efficacy or adverse effects. Studies failing to meet the above inclusion criteria would be excluded from the meta-analysis.
Quality assessment
An open assessment of trials was performed by using the Newcastle-Ottawa quality assessment scale, which assessed trials from the aspects of selection (0–4 points), comparability (0–3 points) and outcome (0–2 points) (16).
Data extraction
Two reviewers independently extracted data from the included articles, based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Any discrepancies were resolved by consensus. For each study, the following data were collected: name of the first author; year of publication; study category; previous TKIs treatment; number of EGFR mutated patients; EGFR mutation type; outcomes of therapeutic efficacy and adverse effects.
Outcome and statistical analysis
The outcomes adopted were: (I) ORR (objective response rate); (II) DCR (disease controlled rate); (III) PFS (progression-free survival); (IV) OS (overall survival); and (V) primary grade 3 or 4 adverse events (AEs). Statistical analyses of ORR, DCR, 6 m-PFS rate, 1 y-PFS rate, 6 m-OS rate and grade 3 or 4 AEs were pooled with the corresponding 95% confidence interval (CI) using R software version 3.2.5 (http://cran.r-project.org/).When OS and PFS could not be extracted from the original reports directly in some trials, the data were deciphered from the survival curves as reported. In the trial by Martin Schuler [2014], time to treatment failure (TTF) was adopted as endpoint, so we regarded TTF approximately as PFS for the minor variation. The heterogeneity between trials was estimated by using inconsistency statistic (I2). Heterogeneity was considered significant when P<0.05. Random effect model was used if heterogeneity existed. Otherwise, a fixed effect model was adopted. Sources of heterogeneity were evaluated by sensitivity analysis, based on the characteristics of EGFR mutation rate. A two-sided P value of <0.05 was considered statistically significant for results.
Results
Included studies
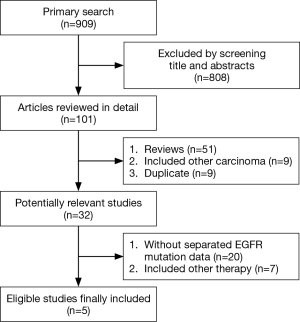
A total number of 545 EGFR-positive patients were available for analysis from five studies (four prospective and one retrospective) after detailed screening from 909 relevant studies (17-21). The selection process was summarized in Figure 1. The baseline characteristics of the included trials were summarized in Table 1. The quality assessment using the Newcastle-Ottawa quality assessment scale showed an average score of 6.8 (range: 0–9). All of the included studies were considered as high-quality studies with scores equal or above “5” (Table 2).

Full table
Full table
Efficacy and toxicity
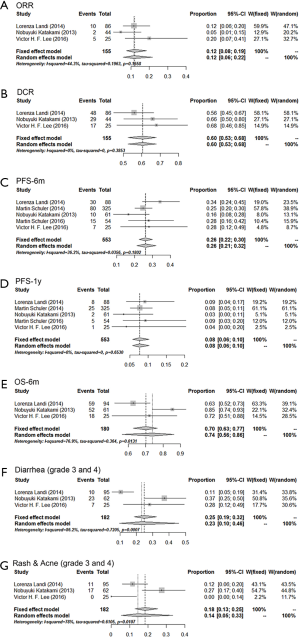
The efficacy of afatinib in EGFR-positive patients after the progression of the first generation EGFR-TKIs was shown in Figure 2. The pooled ORR and DCR were 0.12 (0.08–0.19) and 0.60 (0.53–0.68), respectively. Besides, the 6 m-PFS rate, 1 y-PFS rate and 6 m-OS rate were 0.26 (0.22–0.30), 0.08 (0.06–0.10) and 0.74 (0.56–0.86). Similar to other EGFR-TKIs, diarrhea and skin deformity (rash and acne) were the most common adverse effects of afatinib. The severe adverse effect (grade 3 and 4) rate of diarrhea and skin deformity were 0.23 (0.10–0.46) and 0.14 (0.05–0.33), respectively.
Sensitivity analyses
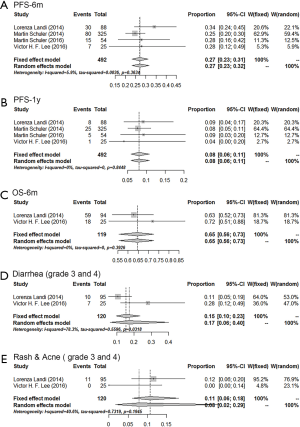
Sensitivity analyses were conducted by excluding the study of Katakami et al. due to the part of extracted data included a small proportion (27.4%) of EGFR-negative/unknown patients, which differed from other studies with 100% EGFR-positive patients. The results of the sensitivity analyses were similar compared to the pooled result using a total of five studies, while the heterogeneity was much smaller (Figure 3). The 6 m-PFS rates, 1 y-PFS rates and 6 m-OS rate were 0.27 (0.23–0.31), 0.08 (0.06–0.11) and 0.65 (0.56–0.73), respectively. Besides, grade 3 and 4 adverse effects rates were 0.17 (0.16–0.40) for diarrhea and 0.11 (0.06–0.18) for rash and acne.
Discussion
This meta-analysis summarized all the present evidence of the potential benefit and toxicity of afatinib therapy after the progression of first-generation EGFR-TKIs for EGFR-positive advanced NSCLC. Pooled data showed 12% for ORR and 60% for DCR, while the pooled 6 m-PFS, 1 y-PFS and 6 m-OS rates were 27%, 8% and 65%, which confirmed the benefits of afatinib therapy after the failure of first generation TKIs for advanced EGFR-positive patients. While severe adverse effects rates of afatinib were 17% for diarrhea and 11% for skin deformity, which were all controllable.
The strategies of subsequent treatments for EGFR-positive patients with acquired resistance of 1st-generation EGFR-TKIs were limited. Chemotherapy has been a common option for those patients. The data of ORR was 18% and median PFS was 4.2 months for patients with chemotherapy alone in the previous study, but the hematologic and neurologic adverse effects were much more common in chemotherapy group (22). Considering the efficacy and toxicity, afatinib could be an optional choice compared with chemotherapy alone with much less adverse effects and slightly lower ORR. Moreover, several randomized controlled trials (RCTs) results showed no clinical benefit on the addition of first-generation TKIs in combination with chemotherapy for those EGFR-positive patients after 1st-generation TKIs failure (23-25). Meanwhile, for advanced EGFR-positive patients with acquired resistance, changing to other 1st generation TKIs seemed to be the inefficient treatment. Recent studies showed the ORR was 6.3% and DCR was 37.5% for EGFR mutated patients using erlotinib after failure of gefitinib, while the DCR of gefitinib administration after failure of erlotinib was 33%. Based on all the evidence above, afatinib seems to be an optional strategy for EGFR-positive patients with acquired resistance of 1st-generation EGFR-TKIs compared with chemotherapy, chemotherapy plus 1st-generation TKIs or alteration of 1st-generation TKIs.
Systematic evaluation of genome-wide analysis showed mainly three different mechanisms for acquired resistance. Most patients (about 50%) showed T790M mutation in EGFR exon 20 after 1st-generation TKIs failure (9). Activation of alternate growth promoting signaling pathways such as PI3K/Akt, c-MET, IGF-R and HER-2 were secondary mechanisms for acquired resistance (26-28). Besides, malignant tissue conversion has been found in a small part of patients with acquired resistance. Recent breakthrough in the TKIs therapy occurs with the development of mutant selective pyrimidine-based third-generation TKIs, typical drugs like AZD9291, WZ4002 and CO-1686, which could irreversibly block T790M mutant EGFR, sparing the wild type (WT) receptor (29). It demonstrated that the ORR of AZD9291 in patients with EGFR T790M positive tumors was 56%, which was higher than that of 12% in terms of afatinib in our meta-analysis outcome. As for the adverse effects, rash and diarrhea occurred in 27% (Grade 3, 0%) and 20% (Grade 3, 1%), respectively (30). It could be concluded that the third generation TKIs may have higher efficacy and less toxicity for patients with acquired 1st-generation TKIs resistance. However, at present, AZD9291 was only approved in USA and Europe, which was unavailable for most patients in other areas unless limited medication from ongoing clinical trials. As a result, at present, afatinib could be a great option for EGFR-positive patients after1st-generation TKIs failure in the area without available third-generation TKIs.
This is the first pooled analysis focused on the efficacy and toxicity of afatinib in advanced EGFR-positive NSCLC patients after failure of 1st-generation TKIs. However, several limitations needed to be considered when interpreting our outcomes. First of all, the sample size of our study was not big because of limited original studies. We also searched for relevant clinical trials from the abstracts of ASCO and ESMO conferences to enrich available data. Secondly, all of the outcome data were obtained from literature review instead of individual patient data, which caused incomplete data for some outcomes. Thirdly, the enrolled study by Katakami et al. included a small proportion of EGFR-negative/unknown patients, which differed from other studies with 100% EGFR-positive patients. Hence, we made sensitivity analyses by excluding the data of this study. Finally, as we all know that 19 Del and 21 L858R were distinct EGFR-positive diseases, subgroup analysis of afatinib usage in the two cohorts respectively was preferred. However, we could not extract relative subgroup data from literature. Therefore, analyses from individual patient data are warranted to solve the above issue.
In conclusion, compared to the chemotherapy, chemotherapy plus 1st-generation TKIs and alteration of 1st-generation TKIs, afatinib seems to be an optional strategy for EGFR-positive patients with acquired resistance of 1st-generation TKIs with higher efficacy and less toxicity. Moreover, afatinib could be a therapeutic option for EGFR-positive patients after1st-generation TKIs failure in the area without available third-generation TKIs.
Acknowledgements
Funding: This work was supported by the National Key R&D Program of China 2016YFC0905500.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013;31:992-1001. [Crossref] [PubMed]
- Souquet PJ, Chauvin F, Boissel JP, et al. Polychemotherapy in advanced non small cell lung cancer: a meta-analysis. Lancet 1993;342:19-21. [Crossref] [PubMed]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005;5:341-54. [Crossref] [PubMed]
- Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012;13:300-8. [Crossref] [PubMed]
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702-11. [Crossref] [PubMed]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Awada AH, Dumez H, Hendlisz A, et al. Phase I study of pulsatile 3-day administration of afatinib (BIBW 2992) in combination with docetaxel in advanced solid tumors. Invest New Drugs 2013;31:734-41. [Crossref] [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [Crossref] [PubMed]
- Pérez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol 2004;22:3238-47. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [Crossref] [PubMed]
- Wells GA, Shea B, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Appl Eng Agric 2014;18:727-34.
- Landi L, Tiseo M, Chiari R, et al. Activity of the EGFR-HER2 dual inhibitor afatinib in EGFR-mutant lung cancer patients with acquired resistance to reversible EGFR tyrosine kinase inhibitors. Clin Lung Cancer 2014;15:411-7.e4. [Crossref] [PubMed]
- Schuler M, Fischer JR, Grohe C, et al. Experience with afatinib in patients with non-small cell lung cancer progressing after clinical benefit from gefitinib and erlotinib. Oncologist 2014;19:1100-9. [Crossref] [PubMed]
- Katakami N, Atagi S, Goto K, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 2013;31:3335-41. [Crossref] [PubMed]
- Schuler M, Yang JC, Park K, et al. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: phase III randomized LUX-Lung 5 trial. Ann Oncol 2016;27:417-23. [Crossref] [PubMed]
- Lee VH, Leung DK, Choy TS, et al. Efficacy and safety of afatinib in Chinese patients with EGFR-mutated metastatic non-small-cell lung cancer (NSCLC) previously responsive to first-generation tyrosine-kinase inhibitors (TKI) and chemotherapy: comparison with historical cohort using erlotinib. BMC Cancer 2016;16:147. [Crossref] [PubMed]
- Goldberg SB, Oxnard GR, Digumarthy S, et al. Chemotherapy with Erlotinib or chemotherapy alone in advanced non-small cell lung cancer with acquired resistance to EGFR tyrosine kinase inhibitors. Oncologist 2013;18:1214-20. [Crossref] [PubMed]
- Xiao BK, Yang JY, Dong JX, et al. Meta-analysis of seven randomized control trials to assess the efficacy and toxicity of combining EGFR-TKI with chemotherapy for patients with advanced NSCLC who failed first-line treatment. Asian Pac J Cancer Prev 2015;16:2915-21. [Crossref] [PubMed]
- Halmos B, Pennell NA, Fu P, et al. Randomized Phase II Trial of Erlotinib Beyond Progression in Advanced Erlotinib-Responsive Non-Small Cell Lung Cancer. Oncologist 2015;20:1298-303. [Crossref] [PubMed]
- Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015;16:990-8. [Crossref] [PubMed]
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. [Crossref] [PubMed]
- Takezawa K, Pirazzoli V, Arcila ME, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov 2012;2:922-33. [Crossref] [PubMed]
- Ohashi K, Sequist LV, Arcila ME, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A 2012;109:E2127-33. [Crossref] [PubMed]
- Finlay MR, Anderton M, Ashton S, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem 2014;57:8249-67. [Crossref] [PubMed]
- Ramalingam S, Yang JC, Lee CK, et al. LBA1_PR: Osimertinib as first-line treatment for EGFR mutation-positive advanced NSCLC: updated efficacy and safety results from two Phase I expansion cohorts. J Thorac Oncol 2016;11:S152. [Crossref] [PubMed]