Supracarinal dissection of the oesophagus and lymphadenectomy by MIE
Since the first report by Dallemagne with the patient in the left lateral position in 1991 and by Cuschieri in the prone position in 1992, minimally invasive oesophagectomy for cancer has gradually become popular and has been performed widely. After learning the technique, the previously undescribed fine anatomy, namely the microanatomy, became obvious under magnified view obtained by positioning the camera at close vicinity to the dissection and the thoracoscopic surgeon’s knowledge of the layer structure in the mediastinum became profounder. Reducing surgical trauma in the mediastinum by rational dissection along the anatomical layers is an important factor in minimally invasive surgery together with reducing the thoracic wound.
Three-field lymphadenectomy has been performed routinely since the mid-1980s in Japan (1), but the extent of lymph node dissection is still in discussion. According to the Efficacy Index [the incidence of metastasis to a region (%), multiplied by the 5-year survival rate (%) of patients with metastasis to that region and divided by 100] (2), the upper mediastinal nodes, such as the bilateral recurrent nodes and tracheobronchial nodes, should be dissected precisely, although dissection of these nodes requires substantial effort by surgeons and is associated with a risk of postoperative complications. The sensitivity for diagnosing the presence of metastasis in each lymph node station is low (3); therefore when retrieving nodes likely being metastasized, there is no excuse for omitting dissection of all nodes. In this chapter, the upper mediastinal microanatomy, which is essential for precise dissection through thoracoscopy, will be demonstrated.
As the left lateral position has been the preferred approach since introduction of thoracoscopy at our institute (4), all figures shown here were obtained from patients in the left lateral position (the upper and left sides correspond with the ventral and cranial aspects, respectively). In order to correspond with monitor images obtained from patients in the prone position, the figures need to be rotated 180 degrees to the right.
Indication for video-assisted thoracoscopic surgery (VATS)
Table 1 shows our indication for VATS. Principally, we do not indicate VATS for patients who received radiation, because mediastinal fibrosis caused by radiation makes microanatomy obscure.

Full table
Principle of thoracoscopic dissection and layer structures in the upper mediastinum
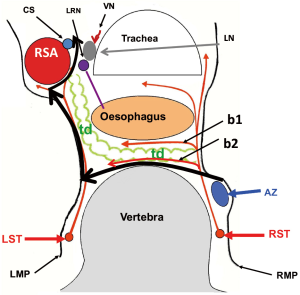
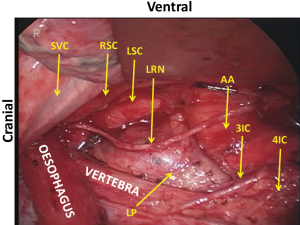
Figure 1 demonstrates the layer structure of the upper mediastinum. The outermost structure under the mediastinal pleura consists of the neural branches. The sympathetic branches from the right trunk dominate over the left and surround the oesophagus and thoracic duct. Black heavy arrows indicate our layer of dissection for total mobilization in the upper mediastinum. Almost all structures divided in the mediastinal mobilization run transversally, except the oesophagus, vagal nerves, and thoracic duct. Therefore, mobilization should be done transversally or orthogonally to the aorta and tracheobronchus. Identifying the structures under magnified view, the neural branches are divided without sealing to avoid the use of energy devices and unnecessary tissue damage. Under magnified view, the epineurium of the recurrent and vagal nerves can be identified easily as shiny fine membrane with fine vessels running longitudinally (Figure 2). As no vessel penetrates the epineurium in the dissection field, exposing the epineurium is the ideal layer of dissection. Under magnified view, the tiny lymph node structure is clearly visible. Histologically, only the afferent lymphatic vessels are located on the convex capsule of the lymph node (Figure 3), while the artery and vasoactive unmyelinated nerve, and the vein and efferent lymphatic vessel are located at the hilum. These hilar structures serve to fix the node in place. In other words, each node has its own direction of hilar fixation. An understanding of the direction of the fixation facilitates nodal dissection (Figure 4).
Dissection of the right recurrent nodes
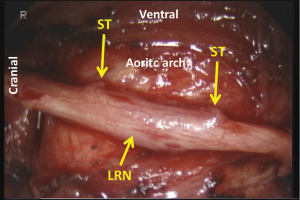
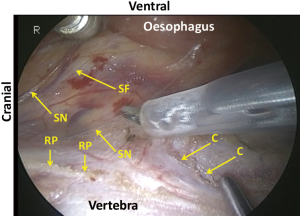
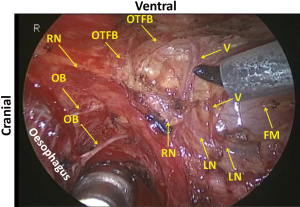
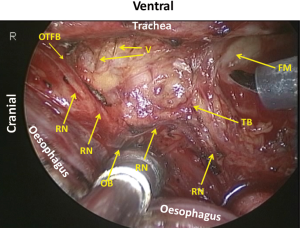
First, the mediastinal pleura are incised along the right vagal nerve, the right subclavian artery, and the ventral margin of the vertebra. Dividing the oesophagotracheal artery, arising from the right subclavian artery and running on the right side of the oesophagus to the anterior aspect of the trachea, at the anterior edge of the vertebra, the fatty tissue consisting of the recurrent nodes is mobilized. Then the epineurium of the vagal nerve is exposed and the right recurrent nerve is identified at its recurring point (just caudal to the right subclavian artery) (Figure 5). The dissection along the recurrent nerve is carried out by exposing the epineurium and dividing the oesophageal branches (commonly 4 or 5 esophageal branches are divided) to the caudal border of the right lobe of the thyroid gland. The nodes present dorsal to the recurrent nerve. The recurrent nerve should be carefully differentiated from the sympathetic nerve from the cervical ganglion (Figure 6). The sympathetic nerve runs along the right subclavian artery, through the arch of the recurrent nerve, and to the frontal aspect of the trachea, and forms a V shape together with the vagal nerve, while in contrast the recurrent nerve forms a U shape. In some patients, the tracheoesophageal artery branches off proximal to the subclavian artery, near the recurrent nerve (Figure 7). In these patients, care should be taken not to injure the artery, so to avoid incurring palsy of the nerve. Depend on each patient’s anatomy, the right inferior thyroidal artery can be recognized (Figure 8).
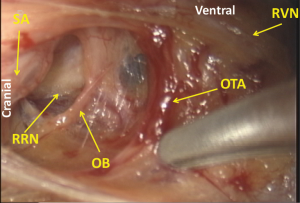
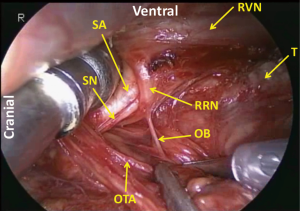
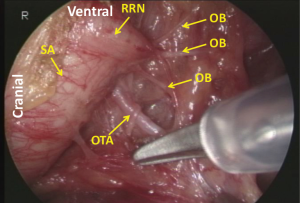
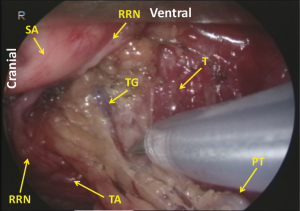
Mobilization of the dorsal aspect of the esophagus
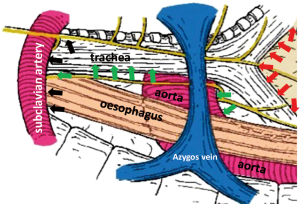
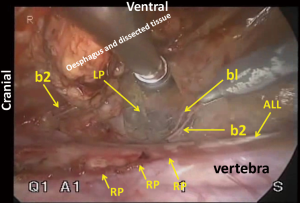
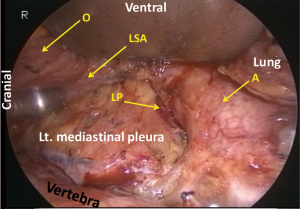
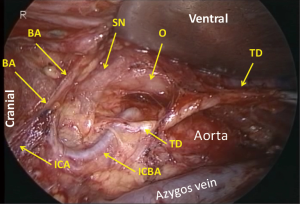
Cranial to the aortic arch, the dorsal aspect of the oesophagus is rather avascular and anatomically simple. However, there can be three planes of dissection according to the right sympathetic branches (Figure 9). When the thoracic duct is preserved, dissection should be conducted along b1 in Figure 1. For total mediastinal mobilization, b2 in Figure 1 is excised and the branches of the left sympathetic trunk are cut (Figure 10), then the left mediastinal pleura are exposed (Figure 11). The azygos arch is mobilized and divided following double ligation. The ligated ends are retracted through the chest wall ventrally and dorsally to enhance mediastinal exposure. The pleura is then incised along the anterior edge of the vertebral column dorsally and the right bronchial artery is doubly clipped and divided at its root as it bifurcates from the intercostobrachial artery (third intercostal artery) (Figure 12). Dissection is continued exposing the ventral aspect of the intercostobrachial artery as far as the right wall of the aortic arch. Then the right wall of the aortic arch is exposed. Cranial to the aortic arch, dissection is carried out ventrally, exposing the left mediastinal pleura, until pulsation of the left subclavian artery is recognized. Because the thoracic duct is covered with this fibrous membrane, the fibrous membrane should be divided for combined resection of the duct (Figure 13). The stump of thoracic duct shows particular appearance because of its intramural smooth muscle (Figure 14). At the level of the pulmonary hilum, the lymphatic collecting ducts from the chest wall and the mediastinum, draining into the thoracic duct, are observed (Figure 15).
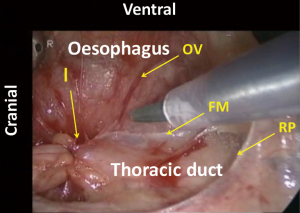
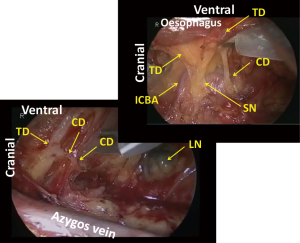
Mobilization of the ventral aspect of the esophagus
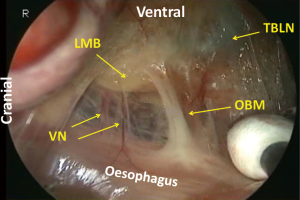
The right vagal nerve is divided at the level of the tracheal bifurcation, just caudal to the pulmonary branches. The oesophagus is mobilized from the trachea by dividing the neural and vascular communication between the bilateral edges of the tracheal cartilage and the oesophagus (Figure 16). There is no vascular communication between the membranous part of the trachea and the oesophagus. At the level of the tracheal bifurcation, the oesophagus makes contact with the membranous part of the left main bronchus and is kept in place by the left vagal nerve and branches of the bronchial artery coming from the left side of the oesophagus. Under magnified view, the oesophagus is found to be fixed in place by the muscular structure. A bundle of the longitudinal muscle of the oesophagus separates from the wall, runs cranially and enters on the left edge of the cartilage part of the tracheobronchus (the oesophagotracheal muscle) (Figure 17).
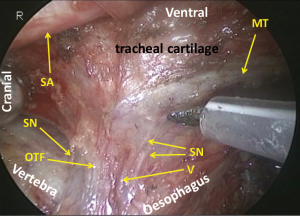
Dissection of the left recurrent nodes
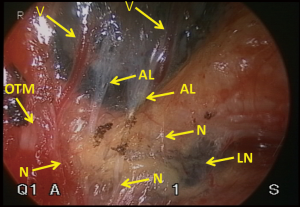
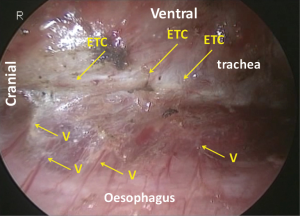
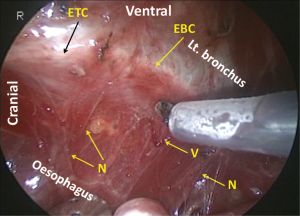
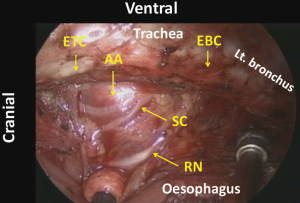
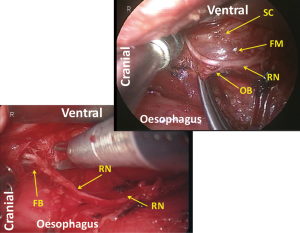
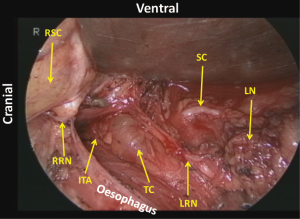
Following mobilization of the dorsal and left aspects of the oesophagus, the tracheobronchus is retracted ventrally to separate it from the now dorsally retracted oesophagus. The right oesophagotracheal fibrous band is excised and the trachea is gradually retracted ventrally and rotated to the left applying a retractor on the right edge of the tracheal cartilage in order to expose the left side. Then the left oesophagotracheal fibrous band is excised (Figure 18), and with the aid of an angulated camera and progressive dorsal retraction of the oesophagus, the dissection is continued on the left side of the cartilage part of the trachea where the fine pretracheal branches of the left recurrent laryngeal nerve are cut (Figure 19). As a result, the sympathetic cardiac branches from the cervical ganglion can be recognized under the fine membrane (Figures 1,20). Because there are no vessels penetrating this fine membrane, mobilization of the tissue from this membrane can be performed bluntly without any bleeding. Following this mobilization, the left recurrent laryngeal nerve together with its surrounding lymph nodes can be retracted dorsally by retracting the oesophagus and applying traction on the oesophageal branches of the nerve (Figures 21,22). This improves the exposure which facilitates further cranial dissection. Superiorly in the neck, several fine branches arising from the left recurrent laryngeal nerve give this area a characteristic appearance like a rake signifying the upper limit of the thoracic dissection. Finally, the left recurrent nerve is separated from the tissue including the lymph nodes and the oesophagus by dividing 5 to 10 of its oesophageal branches. For safe and complete isolation of the nerve, its epineurium (Figures 2,23), which appears glossy with fine vessels running longitudinally, should be exposed. After total isolation of the left recurrent laryngeal nerve, the left side of the lymphatic tissue is separated by exposing the left subclavian artery and dividing the thoracic duct as it approaches the left subclavian artery. Cranial border of dissection along the left recurrent nerve from the chest is at the caudal pole of the thyroid gland, similar as on the right side (Figure 24). Overall, anatomical boundaries for the dissection of the left recurrent laryngeal lymph nodes include the left side of the cartilage part of the trachea, cardiac branches of the sympathetic nerve, the left subclavian artery and the left mediastinal pleura where en-bloc resection without direct traction on the recurrent laryngeal nerve is the main surgical principle (Figure 25).
Efficacy of MIE
Recent population-based analyses revealed that slightly more than 30% of cases of oesophagectomy for cancer are performed thoracoscopically and the percentage is increasing (5-7). However, there is insufficient high-level evidence justifying the thoracoscopic approach. Only one prospective randomized controlled trial (8) and five meta-analyses (9-13) evaluating the benefits of thoracoscopy over open surgery have been reported. A British study demonstrated that there was no significant difference in outcomes between the minimally invasive group and the open surgery group, except for the former having a significantly higher risk of reintervention with increasing odds ratio with every progressive study year (5). Another study from Japan revealed that, compared with open surgery, minimally invasive oesophagectomy was associated with a higher incidence of overall morbidities (40.8% vs. 44.3%), anastomotic leakage (12.5% vs. 14.9%), and reintervention (5.6% vs. 8.0%) (6). Thoracoscopic oesophagectomies done by surgeons in the learning phase which are inevitably included in population-based analyses might be the cause of variance with the other studies. The operative and in-hospital mortality after oesophagectomy is conversely related with hospital volume (14) and the same result is seen after thoracoscopic oesophagectomy. The outcome of oesophagectomy strongly depends on the surgeon’s experience. When it is performed thoracoscopically, additional experience and skill are required of surgeons (15).
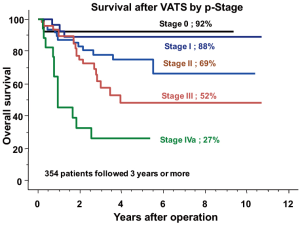
No oncologic adverse effects were detected in meta-analyses (9-13). The thoracoscopic approach did not negatively affect the quality of mediastinal dissection, retrieval of mediastinal nodes, or survival. In our experience, survival after thoracoscopic oesophagectomy was 92%, 88%, 69%, 52% and 27% at 5 years for pStage 0, 1, 2, 3, and 4, respectively (Figure 26). The indication was the same as that for open surgery and perioperative treatment consisted of neoadjuvant and/or adjuvant chemotherapy. Although the data are retrospective, survival was favorable compared with that after open oesophagectomy and similar to that for gastric cancer. However, the evidence of oncologic superiority of thoracoscopy over open surgery is still being evaluated with pragmatic randomized controlled trials.
The quality of dissection has improved with increased understanding of the mediastinal anatomy in vivo under magnified view. The novel anatomical knowledge enhanced through thoracoscopy can serve as feedback for open surgery to improve the quality of mediastinal dissection. In this chapter, the microanatomy usually recognized in the treatment of non radiated patients is presented. It is supposed that in patients undergoing neoadjuvant treatment, especially radiation, the fine anatomy may become obscure because of mediastinal fibrosis. Even so, understanding of the innate microanatomy is essential to ultimately perform the ideal oesophagectomy, even in patients after neoadjuvant therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fujita H. History of lymphadenectomy for esophageal cancer and the future prospects for esophageal cancer surgery. Surg Today 2015;45:140-9. [Crossref] [PubMed]
- Udagawa H, Ueno M, Shinohara H, et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 2012;106:742-7. [Crossref] [PubMed]
- Funai T, Osugi H, Higashino M, et al. Estimation of lymph node metastasis by size in patients with intrathoracic oesophageal cancer. Br J Surg 2000;87:1234-9. [Crossref] [PubMed]
- Osugi H, Takemura M, Higashino M, et al. Video-assisted thoracoscopic esophagectomy and radical lymph node dissection for esophageal cancer. A series of 75 cases. Surg Endosc 2002;16:1588-93. [Crossref] [PubMed]
- Mamidanna R, Bottle A, Aylin P, et al. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg 2012;255:197-203. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259-66. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Yang CJ, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 2009;64:121-33. [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci 2010;55:3031-40. [Crossref] [PubMed]
- Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg 2012;147:768-76. [Crossref] [PubMed]
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. [Crossref] [PubMed]
- Fujita H, Ozawa S, Kuwano H, et al. Esophagectomy for cancer: clinical concerns support centralizing operations within the larger hospitals. Dis Esophagus 2010;23:145-52. [Crossref] [PubMed]
- Osugi H, Takemura M, Higashino M, et al. Learning curve of video-assisted thoracoscopic esophagectomy and extensive lymphadenectomy for squamous cell cancer of the thoracic esophagus and results. Surg Endosc 2003;17:515-9. [Crossref] [PubMed]


