Endoscopic diagnosis and management of early squamous cell carcinoma of esophagus
Introduction
Squamous cell carcinoma (SCC) remains the most common histological subtype of esophageal cancers in Asia, in particular China and Japan. The disease is associated with poor prognosis and most patients were diagnosed at a late stage when curative treatment is no longer possible. For patients with localized disease, surgery provides a chance of cure but is also associated with significant surgical morbidity and mortality. Much progress has been made in the past decade to improve endoscopic detection of early esophageal cancers. Potential curative endoscopic therapy has also been developed to reduce the morbidity associated with the treatment for esophageal cancers. This article aims to provide an updated review on the latest development of endoscopic diagnosis and treatment of early esophageal SCC.
Endoscopic detection and diagnosis of esophageal SCC
Conventional white light imaging (WLI) endoscopy with endoluminal biopsy has been the gold standard for detection and diagnosis of esophageal cancers. For patients presenting with symptoms such as dysphagia, the tumors are likely of significant size and conventional WLE would be adequate for diagnosis. However, when the endoscopy was performed as a screening or surveillance, the sensitivity of WLE in detecting early lesions would be much lower.
Chromoendoscopy with Lugol’s iodine has been utilized as the preferred method of screening in high-risk patients since early 2000s. The agent stains to glycogen in normal squamous epithelium, giving off its brown color under white light endoscopy. In glycogen depleted epithelium such as dysplasia, the mucosa would appear “unstained”. In one early prospective study of 225 adults from Linxian, China who suffered from esophageal dysplasia or carcinoma, unstained mucosal areas after iodine application had sensitivities of 63%, 93%, 96%, and 100% for identifying mild, moderate, severe dysplasia and early invasive carcinoma, respectively (1). Its use among patients with head and neck cancers had been validated in multiple prospective studies (2-4). However, the use of Lugol’s iodine is associated with a number of problems. First, the solution irritates the esophageal mucosa and can cause chest pain or discomfort. It could also cause hypersensitivity reaction, leading to mucosal damage of the esophagus and stomach (5-8). Second, Lugol chromoendoscopy has low specificity for esophageal neoplasia, leading to a high false positive rate and the need for unnecessary biopsies (1-4). The need for application of the dye also would also potentially increase the procedural time.
Narrow band imaging (NBI) technology was introduced in the early 2000 to facilitate endoscopic diagnosis of gastrointestinal lesions. By using filter of two specific peak wavelengths (415 and 540 nm), the mucosal surface and vascular pattern of the gastrointestinal tract could be enhanced, allowing endoscopists to detect and characterize lesions (9). The system is incorporated now with ordinary endoscopes and could be easily activated by pressing a button. Two different approaches of utilizing NBI technology have been described for screening of esophageal lesions: the non-magnifying endoscopy for detection of lesion and the combination of magnifying endoscopy for characterization of these lesions.
Using non-magnifying NBI endoscopy, normal esophageal mucosa would appear green in color, while in the presence of lesions there would be brownish discoloration. This is an invaluable tool for screening of abnormal lesions in the esophagus as well as the hypopharyngeal area. The NBI mode could be switched on when the endoscope is inserted into the oral cavity. Upon passage of the upper esophageal sphincter examination of the esophagus could be completed without changing of the mode. Conventional white light endoscopic examination of the stomach is currently still the gold standard due to the limitation of the brightness with the NBI technology. After complete examination of the stomach, the esophagus could be examined again using WLI. However, at the level of the cervical esophagus, the NBI mode should be switched on again to avoid missing lesions at this region during scope insertion.
Multiple prospective studies have shown that non-magnified NBI examination is superior to WLI in detection of early esophageal lesions for screening of high-risk patients (10-13). The performance of non-magnified NBI and Lugol chromoendoscopy were similar in these studies. With the addition of magnified endoscopy, characterization of surface vascular pattern by observing the intrapapillary capillary loops (IPCL) would help to increase the accuracy of NBI endoscopy. In a multicenter randomized study by Muto et al, NBI with magnification was compared with WLI as screening modality for patients with head and neck SCC (14). Among 320 enrolled patients, 212 esophageal superficial cancers were detected. NBI with magnifying endoscopy achieved a significantly higher sensitivity (97.2% vs. 55.2%), accuracy (88.9% vs. 56.5%), and NPV (72.8% vs. 20.3%) than WLI endoscopy. A recent meta-analysis including 11 cross sectional studies and 1 randomized study with a total of 1,911 patients, found no difference in sensitivity between NBI and Lugol chromoendoscopy for diagnosing early esophageal cancer (15). In addition, NBI endoscopy also had a higher specificity comparing to Lugol chromoendoscopy (per lesion analysis 82% vs. 37%). Although Lugol chromoendoscopy is still considered as the gold standard, NBI endoscopy should be regarded as a reliable alternative option for screening of early esophageal cancers, with potential additional benefit of less patient discomfort and shorter procedural time.
Evaluation of IPCL
Inoue et al. first reported his observation of esophageal mucosal microvascular pattern utilizing magnifying WLI endoscopy (16,17). A progressive change in the IPCL was also noted with increasing destruction of the mucosa by neoplastic transformation of the esophagus. Characterization of IPCL using WLI is particularly challenging due to poor contrast of the vessels comparing with background pinkish mucosa. The use of NBI greatly facilitates observation of changes in the microvascular pattern of the esophagus by selectively enhancing the brown colored IPCL. According to the original classification, a total of 5 subtypes of IPCL were identified (18,19).
IPCL I & II—normal esophagus or esophagitis
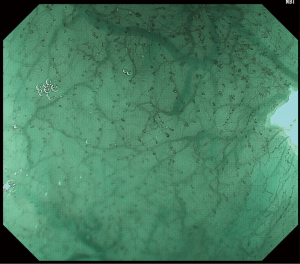
Using NBI endoscopy with magnification, IPCL can be visualized readily as brown colored loops. Occasionally flow of individual red blood cells within the IPCL could be observed as well. In normal esophageal mucosa, there would not be any color change of the mucosa on NBI, i.e., absence of brownish discolored area. The IPCL would appear as small open coiled loops with a diameter of ~7–10 nm (IPCL-I) (Figure 1). With inflammatory change of the esophagus, there would typically be dilatation and elongation of IPCL over the margin of the lesion (IPCL-II).
IPCL III & IV—tissue atypia or early neoplastic change
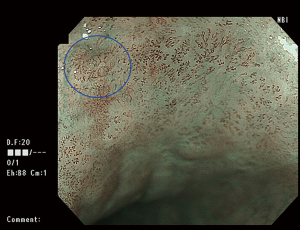
Lesions with brownish discoloration on NBI should be further evaluated with magnifying endoscopy. Those with minimal microvascular proliferation can be categorized as IPCL type III (Figure 2). These lesions are most likely regional atrophic mucosa or low-grade intraepithelial neoplasia, and regular endoscopic surveillance should be performed. IPCL type IV is characterized by dilatation and elongation of the vessels, representing high-grade intraepithelial neoplasia (Figure 3).

IPCL V1–3 and VN—from carcinoma in-situ to submucosal invasive carcinoma
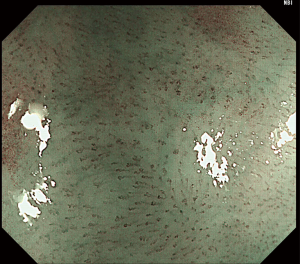
In carcinoma in situ, four characteristic changes of IPCL in the esophageal brown discolored areas have been observed (IPCL V1): dilatation, meandering, caliber change and non-uniformity in the appearance (Figure 4). Progressive destruction of the IPCL would occur in deeper extension of the esophageal carcinoma. In IPCL V2 corresponding to M2 invasive carcinoma, the morphology of IPCL demonstrated additional elongation of the vessels in the vertical plane (Figure 5). IPCL V3 is characterized by loss of the loop configuration of the vessels (Figure 6). On histology, these usually represent M3 to SM1 invasive carcinoma. When large new abnormal vessels are observed (usually >3 times of V3 IPCL), they likely correspond to deep submucosal invasive carcinoma and are classified as IPCL type VN.
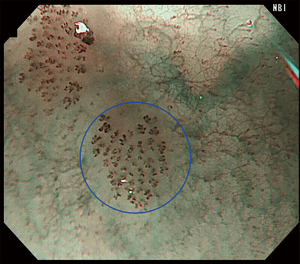
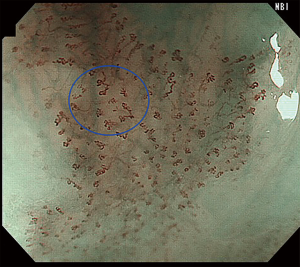
Using the above classification, Sato et al. analyzed 446 lesions from 358 patients with esophageal neoplasia (20). The sensitivity and specificity for IPCL type V1–2 for M1–2 disease was 89.5% and 79.6% respectively. This is an important finding as M1–2 carcinomas are lesions amenable for endoscopic resection, which would be discussed further in this review. A substantial interobserver and intraobserver agreement for the IPCL classification was reported as well, but only three reviewers were involved in the calculation of the kappa value in their study.
On the other hand, Arima et al. proposed another classification based on magnifying endoscopy (21). The vascular patterns were divided into four subtypes. In addition, the concept of avascular areas (AVA) was also introduced, with the larger size AVA representing deeper invasion of the esophageal carcinoma.
In an attempt to avoid multiplicity of classification systems and complicated criteria, the Japanese Esophageal Society (JES) proposed a new classification in 2012 (22). In this new system, morphology of IPCL is classified into type A and B based on the presence of abnormality including weaving, dilatation, irregular caliber, and difference in shape (Figures 1,2). Type B vessels are further subclassified into B1–B3 based on the size of the abnormal IPCL and whether a loop-like appearance is preserved. AVA were also classified into small (<0.5 mm), medium 0.5–3 mm), large size (>3 mm), and further incorporated with the IPCL morphological classification in predicting the depth of invasion (Figures 3-6). A prospective multicenter study was reported using this classification (23). The overall accuracy of the system was 90.5%. The sensitivity and positive predictive value of B1 vessels for M1–M2 tumors were 97.5% and 92.4% respectively, reflecting optimal diagnostic accuracy in deciding for endoscopic resection.
Endoscopic treatment of esophageal SCC
Two prerequisites are required for successful endoscopic treatment of esophageal SCC: complete removal of the primary tumor in the absence of regional lymph node metastasis. In order to achieve that, reliable method of endoscopic resection is mandatory, ideally with en bloc removal of the tumor, as well as an accurate prediction of the risk of lymph node metastasis. In Japan, endoscopists have been performing endoscopic mucosal resection (EMR) for early esophageal cancers for disease confined to the mucosa since the 1990s. In a large nationwide study of 2,418 patients with early esophageal cancers, the risks of lymph node metastasis were 0% and 3.3% for M1 (disease confined to epithelium) and M2 (disease confined to lamina propria mucosa) respectively (24). Tumors invading to muscularis mucosae (M3) or superficial third of submucosa (SM1) had a much higher risk of lymph node metastasis at 10.2% and 26.5%. In another study of 240 surgically resected early carcinomas, tumors that invade beyond lamina propria (M3 & SM1) had no lymph node metastasis if there was absence of lymphovascular permeation, vertical tumor invasion <200 μm and tumor grading of 1 or 2 (25). As a result, endoscopic resection has been recommended only for SCC confined to M1 or M2 level (absolute indication). M3 or SM1 tumors <200 μm are considered relative indications if there is no clinical evidence of lymph node metastasis (26).
EMR involves the use of endoscopic snare for resection of a lesion usually after artificially raising the lesion with submucosal injection of a mixed solution. Various techniques have been used to facilitate the EMR procedure, such as the band assisted or cap assisted techniques. The major limitation of EMR lies in the difficulty in achieving en bloc resection for larger size lesions. In the aforementioned nationwide study, piecemeal resection was required in 94% of the cases if the tumor diameter is larger than 2 cm (24). Pathological assessment of the resected tumor becomes inaccurate if tumors are resected in piecemeal manner, in particular determination of margin clearance and the depth of invasion. Moreover, residual tumor could be left at the edge of each snare application during piecemeal EMR and led to an increased risk of local recurrence (27).
Endoscopic submucosal dissection (ESD)
ESD is an endoscopic technique initially developed for resection of gastric neoplasms (28-30). Compared with EMR where lesion size is the main factor in determining the need for piecemeal resection, ESD could achieve en bloc resection regardless of the lesion size and is also less affected by fibrosis in the submucosal layer. The technique of ESD has now been extended to the rest of the gastrointestinal tract including early esophageal neoplasia. Compared to gastric ESD, esophageal ESD is more difficult to perform due to narrow space in the lumen as well as a higher risk of perforation owing to a thin muscular layer. Favorable outcomes have been reported and will be elaborated further below.
Esophageal ESD could be performed under conscious sedation or general anaesthesia. Generally, we prefer procedure under general anaesthesia especially for cases with expected long duration and lesions locating in the proximal esophagus as the risk of perforation significantly increase if the patient could not cooperate well during conscious sedation. Special endoscopic electrosurgical knives are required during the ESD procedures. These are specially designed devices for precise tissue cutting and hemostasis. Two types of knives have been developed: the non-insulated and the insulated tip knives. In our ESD procedures we usually use the Dual Knife J (KD655Q, Olympus Medical Systems, Tokyo, Japan), a type of non-insulated knife with a knob-shaped tip and injection port. A high definition endoscope with water-jet function and a transparent hood mounted at the tip is preferred. Esophageal ESD involves four steps: Marking, lifting, incision and dissection. Precise marking of the margin of the lesion is imperative as once the lesion is lifted the margins would become indistinct. Next, lifting of the lesion is performed by submucosal injection of a mixed solution. Normal saline, hyaluronic acid or glycerin solution have all been used for injection, with the addition of adrenaline and indigo carmine as a dye to highlight the submucosal plane. Circumferential mucosal incision would then be performed, usually from the anal side of the lesion. Particular attention has to be made with regard to the effect of gravity, as pooling of fluid in the dependent area could significantly obscure the endoscopic view. After mucosal incision, complete submucosal dissection could be performed by clearly visualizing the submucosal plane between the mucosa and the muscularis propria. Various retraction methods have been reported to facilitate dissection. The “clip traction” method is one of the easiest techniques reported (31,32). It involves the use of a long thread of suture tied to an endoscopic clip, which is applied on the oral side of the lesion after mucosal incision and the suture retrieved in the mouth. Upon pulling of the suture externally, countertraction could be achieved for better exposure of the submucosal plane. A shorter duration of procedure using the “clip traction” method was required compared to conventional ESD (33). Careful hemostasis is needed to avoid reactionary and delayed hemorrhage. Large submucosal vessels encountered during dissection could be coagulated with the electrosurgical knives or hemostatic forceps (Coagrasper, FD-410LR, Olympus Medical Systems, Tokyo, Japan). Resected specimen should be pinned on a block fixed in formalin for dedicated pathological assessment.
Outcomes of endoscopic resection of early esophageal cancers
Early reports on clinical outcomes of esophageal ESD have been promising with a high en bloc resection rate of 95–100% and a low complication rate (Bleeding 0%, perforation 3–6%) (34-36). In a recent meta-analysis of 8 comparative studies between esophageal ESD and EMR, ESD achieved a significant higher rate of en bloc resection (odds ratio =52.8, 95% CI: 25.6–108.8) but at a higher risk of perforation (odds ratio =2.19, 95% CI: 1.08–4.47) (37). A longer procedural time was required with ESD. Risk of local recurrence was significantly lower with ESD when compared to EMR (0.3% versus 11.5%; odds ratio =0.08, 95% CI: 0.03–0.23). Ono et al. reported the long-term outcomes of esophageal ESD of 84 patients with early squamous cell cancers (36). The 5-year cause-specific survival was 100% for M1–M2 carcinomas and 85% for M3/SM1 invasive carcinomas. A comparable cause specific survival at 5 years was also reported in an earlier study between conventional EMR and surgery for M3/SM1 carcinomas (95% and 93.5%) (38).
In recent years, post-procedural strictures have become one of the major concerns for esophageal ESD. Studies with multivariate analysis have identified dissection of >3/4 circumference of the lumen as the most important risk factor for occurrence of such complication (39-41). Risk of stricture after near circumferential ESD could be as high as 100%. Numerous preventive strategies have been proposed, including the use of topical or systemic anti-inflammatory agents, prophylactic endoscopic balloon dilation and tissue engineering approaches (42-46). Unfortunately, the efficacy of these strategies is not well established, and there is currently a lack of standardized approach in prevention of this potentially debilitating complication.
Conclusions
In the recent decade, numerous advances have been made in accurate endoscopic diagnosis of early esophageal SCC, as well as the advent of novel endoscopic approach in curative resection of such lesions. With increased in detection and endoscopic resection of early esophageal carcinoma, patients suffering from this traditionally lethal disease could hopefully enjoy an extended survival with improved quality of life.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer 1998;83:220-31. [Crossref] [PubMed]
- Shiozaki H, Tahara H, Kobayashi K, et al. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer 1990;66:2068-71. [Crossref] [PubMed]
- Dubuc J, Legoux J, Winnock M, et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy 2006;38:690-5. [Crossref] [PubMed]
- Hashimoto CL, Iriya K, Baba ER, et al. Lugol's dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol 2005;100:275-82. [Crossref] [PubMed]
- Sreedharan A, Rembacken BJ, Rotimi O. Acute toxic gastric mucosal damage induced by Lugol's iodine spray during chromoendoscopy. Gut 2005;54:886-7. [Crossref] [PubMed]
- Park JM, Seok Lee I, Young Kang J, et al. Acute esophageal and gastric injury: complication of Lugol's solution. Scand J Gastroenterol 2007;42:135-7. [Crossref] [PubMed]
- Thuler FP, de Paulo GA, Ferrari AP. Chemical esophagitis after chromoendoscopy with Lugol's solution for esophageal cancer: case report. Gastrointest Endosc 2004;59:925-6. [Crossref] [PubMed]
- Kondo H, Fukuda H, Ono H, et al. Sodium thiosulfate solution spray for relief of irritation caused by Lugol's stain in chromoendoscopy. Gastrointest Endosc 2001;53:199-202. [Crossref] [PubMed]
- Gono K, Yamazaki K, Doguchi N, et al. Endoscopic Observation of Tissue by Narrowband Illumination. Optical Review 2003;10:211-5. [Crossref]
- Lecleire S, Antonietti M, Iwanicki-Caron I, et al. Lugol chromo-endoscopy versus narrow band imaging for endoscopic screening of esophageal squamous-cell carcinoma in patients with a history of cured esophageal cancer: a feasibility study. Dis Esophagus 2011;24:418-22. [Crossref] [PubMed]
- Ide E, Maluf-Filho F, Chaves DM, et al. Narrow-band imaging without magnification for detecting early esophageal squamous cell carcinoma. World J Gastroenterol 2011;17:4408-13. [Crossref] [PubMed]
- Yokoyama A, Ichimasa K, Ishiguro T, et al. Is it proper to use non-magnified narrow-band imaging for esophageal neoplasia screening? Japanese single-center, prospective study. Dig Endosc 2012;24:412-8. [Crossref] [PubMed]
- Kawai T, Takagi Y, Yamamoto K, et al. Narrow-band imaging on screening of esophageal lesions using an ultrathin transnasal endoscopy. J Gastroenterol Hepatol 2012;27 Suppl 3:34-9. [Crossref] [PubMed]
- Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol 2010;28:1566-72. [Crossref] [PubMed]
- Morita FH, Bernardo WM, Ide E, et al. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer 2017;17:54. [Crossref] [PubMed]
- Inoue H, Honda T, Yoshida T, et al. Ultra-high Magnification Endoscopy of the Normal Esophageal Mucosa. Dig Endosc 1996;8:134-8. [Crossref]
- Inoue H, Honda T, Nagai K, et al. Ultra-high Magnification Endoscopic Observation of Carcinoma in situ of the Esophagus. Dig Endosc 1997;9:16-8. [Crossref]
- Inoue H. Magnification endoscopy in the esophagus and stomach. Digestive Endoscopy 2001;13:S40-1. [Crossref]
- Inoue H, Kaga M, Ikeda H, et al. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Ann Gastroenterol 2015;28:41-8. [PubMed]
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015;47:122-8. [Crossref] [PubMed]
- Arima M, Tada M, Arima H. Evaluation of microvascular patterns of superficial esophageal cancers by magnifying endoscopy. Esophagus 2005;2:191-7. [Crossref]
- Oyama T, Momma K, Makuuchi H. Japan esophageal society classification of superficial esophageal squamous cell carcinoma. Endosc Dig 2012;24:466-8.
- Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus 2017;14:105-12. [Crossref] [PubMed]
- Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery 1998;123:432-9. [Crossref] [PubMed]
- Tajima Y, Nakanishi Y, Ochiai A, et al. Histopathologic findings predicting lymph node metastasis and prognosis of patients with superficial esophageal carcinoma: analysis of 240 surgically resected tumors. Cancer 2000;88:1285-93. [Crossref] [PubMed]
- Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 2015;12:1-30.
- Katada C, Muto M, Manabe T, et al. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005;61:219-25. [Crossref] [PubMed]
- Yamamoto H, Kawata H, Sunada K, et al. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc 2002;56:507-12. [Crossref] [PubMed]
- Yahagi N, Fujishiro M, Kakushima N, et al. Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type). Digestive Endoscopy 2004;16:34-8. [Crossref]
- Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005;17:54-8. [Crossref]
- Ota M, Nakamura T, Hayashi K, et al. Usefulness of clip traction in the early phase of esophageal endoscopic submucosal dissection. Digestive Endoscopy 2012;24:315-8. [Crossref] [PubMed]
- Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc 2012;45:375-8. [Crossref] [PubMed]
- Koike Y, Hirasawa D, Fujita N, et al. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: Randomized controlled trial. Digestive Endoscopy 2015;27:303-9. [Crossref] [PubMed]
- Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3:S67-70. [Crossref] [PubMed]
- Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860-6. [Crossref] [PubMed]
- Guo HM, Zhang XQ, Chen M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol 2014;20:5540-7. [Crossref] [PubMed]
- Shimizu Y, Tsukagoshi H, Fujita M, et al. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc 2002;56:387-90. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009;41:661-5. [Crossref] [PubMed]
- Takahashi H, Arimura Y, Okahara S, et al. Risk of perforation during dilation for esophageal strictures after endoscopic resection in patients with early squamous cell carcinoma. Endoscopy 2011;43:184-9. [Crossref] [PubMed]
- Shi Q, Ju H, Yao LQ, et al. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy 2014;46:640-4. [Crossref] [PubMed]
- Ezoe Y, Muto M, Horimatsu T, et al. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol 2011;45:222-7. [Crossref] [PubMed]
- Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011;74:1389-93. [Crossref] [PubMed]
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011;73:1115-21. [Crossref] [PubMed]
- Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012;143:582-8.e1-2.
- Sakaguchi Y, Tsuji Y, Ono S, et al. Polyglycolic acid sheets with fibrin glue can prevent esophageal stricture after endoscopic submucosal dissection. Endoscopy 2015;47:336-40. [PubMed]