A case report of pulmonary cryptococcosis presenting as endobronchial obstruction
Introduction
Cryptococcal infections are mostly common in immunocompromized patients such as AIDS, organ transplantation, or hematologic malignancy. Pulmonary cryptococcosis usually consists of pulmonary nodules or masses and focal areas of consolidation (1). Cryptococcosis presenting as endobronchial obstruction in immunocompetent patient has rarely been reported (2,3).
Case report
A 44-year-old male patient who presented with a 3-month history of cough, hemoptysis and 7 kg weight loss. He worked as a builder and smoked 40 cigarettes·day–1 for 20 years. Initial investigations revealed a leucocyte count of 8.6×109/L, fasting blood glucose of 5.4 mmol/L, serum albumin of 42 g/L, HIV testing was negative and normal arterial blood gases on room air. Sputum microbiology was negative. The patient was initially prescribed third-generation cephalosporins for pneumonia, but failed to improve.
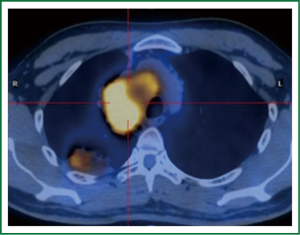
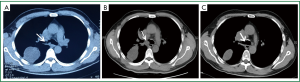
A chest CT scan (Figure 1A) showed a mass, 67.7 mm in diameter in the right upper lobe and 72.4 mm in diameter in the right hilar, with evidence of narrowing of the right upper lobe bronchus. PET-CT (Figure 2) scan showed the mSUVs for FDG uptake of lesions ranged from 9.86 to 10.99.
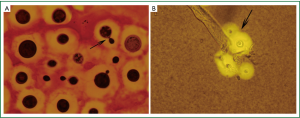
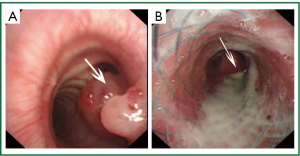
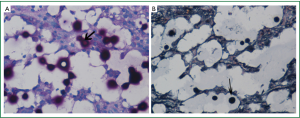
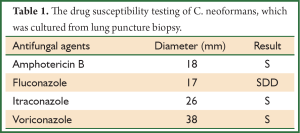
Brochoscopy confirmed a mass over the right main bronchus orifice that caused occlusion (Figure 3). Because of the risk of bleeding, the mass wasn’t biopsied. A biopsy specimen was taken from the mass locating in the posterior segment of the right upper lobe by lung puncture biopsy and showed cryptococcus infection (Figure 4). Culture of lung mass was C. neoformans (Figure 5). The susceptibility was done (Table 1). The serum was positive for cryptococcal antigen, with a titer of more than 1:1,280.
Full Table
Based on these results, we diagnosed his as having endobronchial cryptococcosis. The patient was given itraconazole, then voriconazole for more than one month but failed to improve (Figure 1B). During the period, the shortness of breath increased and a chest CT scan (Figure 1B) showed a mass with evidence of complete occlusion of the right main bronchus. Tracheal endoscopic mass ablation and tracheal stent implantation was done. Following a test dose of 20 mg amphotericin B liposome, given intravenously without systemic reaction, the patient was commenced on 100 mg amphotericin B liposome daily intravenously. After six weeks treatment, a follow-up CT scan (Figure 1C) has showed the mass was obviously absorbed. A repeat bronchoscopy (Figure 3B) confirmed the mass of right main bronchus was disappeared. The patient was discharged after 6 months antifungal therapy.
Discussion
Cryptococcosis typically occurs in immunocompromised patients such as those with HIV/AIDS, the incidence was 5% to 10%, it is estimated that approximately 1,000,000 cases of cryptococcal infection annual in HIV patients (4,5),but it can also occur in immunocompetent patients (6).
The most common radiographic characteristics of pulmonary cryptococcosis consist of solitary or multiple pulmonary nodules or mass, segmental or lobar consolidation, or reticulonodular pattern (7). The infection in immunocompetent hosts shows the most frequent pattern of lung abnormalities was patchy consolidation opacity and solitary pulmonary mass opacity (6,8).
Cryptococcosis presenting as an endobronchial tumor-like growth has rarely been described (3,9). Our case was unique, as the diagnosis of cryptococcosis was based on both histology and culture from multiple sterile samples, including lung and bronchial tissues. The patient had partial clinical and radiological improvement. We could find no evidence of immunocompromise in our patient, and there appears to be insufficient evidence from the literature that presentation with an endobronchial lesion is associated with the status of a patients immune system (10).
PET-CT can help identify tumors and benign lesions, but sixty percent of pulmonary cryptococcosis showed higher FDG uptake and the mSUVs for FDG uptake of lung parenchymal lesions ranged from 1.8 to 9.3 (11).
Early and appropriate antifungal therapy is essential for optimum patient outcomes in cryptococcosis infections. Fuconazole is commonly recommended to treatment of pulmonary cryptococcal disease in immunocompetent patients (12). But in recent years, the potential development of fluconazole resistance poses a threat to the management of cryptococcal disease. The ARTEMIS DISK Global Surveillance Group has identified from 1997-2007 a progressive increase in resistance to fluconazole among isolates of C. neoformans from the time periods 1997 to 2000 (7.3%), 2001 to 2004 (10.9%), and 2005 to 2007 (11.7%). Especially, in the Asia-Pacific region, fluconazole resistance among C. neoformans isolates increased from 5.1% to 22.6% over the 7-year period (13). The reasons of C. neoformans emerging resistance to fluconazole were not only the overuse of prophylaxis such as for oral candidiasis in immunocompromised patients, the widespread use of the antifungal as primary and maintenance therapy, but a potential alternative mechanism of heteroresistance (14).
Although no particular pattern of cross-resistance to other azoles was observed in the fluconazole-resistant cases, treatment was still relapsed even if voriconazole replace the fluconazole (15,16). Subsequent use of amphotericin B therapy in the majority of cases appears to have brought about their clinical recovery (16). Further studies that were fail to be treated with fluconazole would provide better therapy options and explore the reasons for poor treatment of fluconazole.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fox DL, Müller NL. Pulmonary cryptococcosis in immunocompetent patients: CT findings in 12 patients. AJR Am J Roentgenol 2005;185:622-6. [PubMed]
- Inoue Y, Miyazaki Y, Izumikawa K, et al. Pulmonary cryptococcosis presenting as endobronchial lesions in a patient under corticosteroid treatment. Intern Med 2007;46:519-23. [PubMed]
- Thomas R, Christopher DJ, Balamugesh T, et al. Endobronchial pulmonary cryptococcosis and tuberculosis in an immunocompetent host. Singapore Med J 2012;53:e32-4. [PubMed]
- Patel S, Shin GY, Wijewardana I, et al. The prevalence of cryptococcal antigenemia in newly diagnosed HIV patients in a Southwest London cohort. J Infect 2013;66:75-9. [PubMed]
- Park BJ, Wannemuehler KA, Marston BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009;23:525-30. [PubMed]
- Ye F, Xie JX, Zeng QS, et al. Retrospective analysis of 76 immunocompetent patients with primary pulmonary crypotococcosis. Lung 2012;190:339-46. [PubMed]
- Woodring JH, Ciporkin G, Lee C, et al. Pulmonary cryptococcosis. Semin Roentgenol 1996;31:67-75. [PubMed]
- Zhang PH, Hu BJ, He LX, et al. The characteristics of CT imaging and diagnosis of pulmonary cryptococcosis in 42 cases with non-acquired immune deficiency syndrome. Zhonghua Nei Ke Za Zhi 2009;48:362-6. [PubMed]
- Inoue Y, Miyazaki Y, Izumikawa K, et al. Pulmonary cryptococcosis presenting as endobronchial lesions in a patient under corticosteroid treatment. Intern Med 2007;46:519-23. [PubMed]
- Chang YS, Chou KC, Wang PC, et al. Primary pulmonary cryptococcosis presenting as endobronchial tumor with left upper lobe collapse. J Chin Med Assoc 2005;68:33-6. [PubMed]
- Song KD, Lee KS, Chung MP, et al. Pulmonary cryptococcosis: imaging findings in 23 non-AIDS patients. Korean J Radiol 2010;11:407-16. [PubMed]
- Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis 2010;50:291-322. [PubMed]
- Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: 10.5-year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol 2009;47:117-23. [PubMed]
- Varma A, Kwon-Chung KJ. Heteroresistance of Cryptococcus gattii to fluconazole. Antimicrob Agents Chemother 2010;54:2303-11. [PubMed]
- Paugam A, Dupouy-Camet J, Blanche P, et al. Increased fluconazole resistance of Cryptococcus neoformans isolated from a patient with AIDS and recurrent meningitis. Clin Infect Dis 1994;19:975-6. [PubMed]
- Cheong JW, McCormack J. Fluconazole resistance in cryptococcal disease: emerging or intrinsic? Med Mycol 2013;51:261-9. [PubMed]